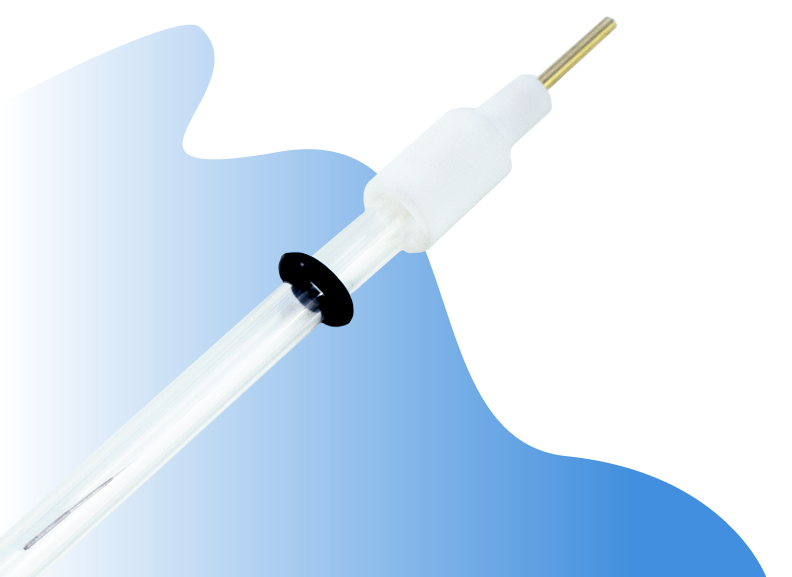
How To Prepare a Reference Electrode
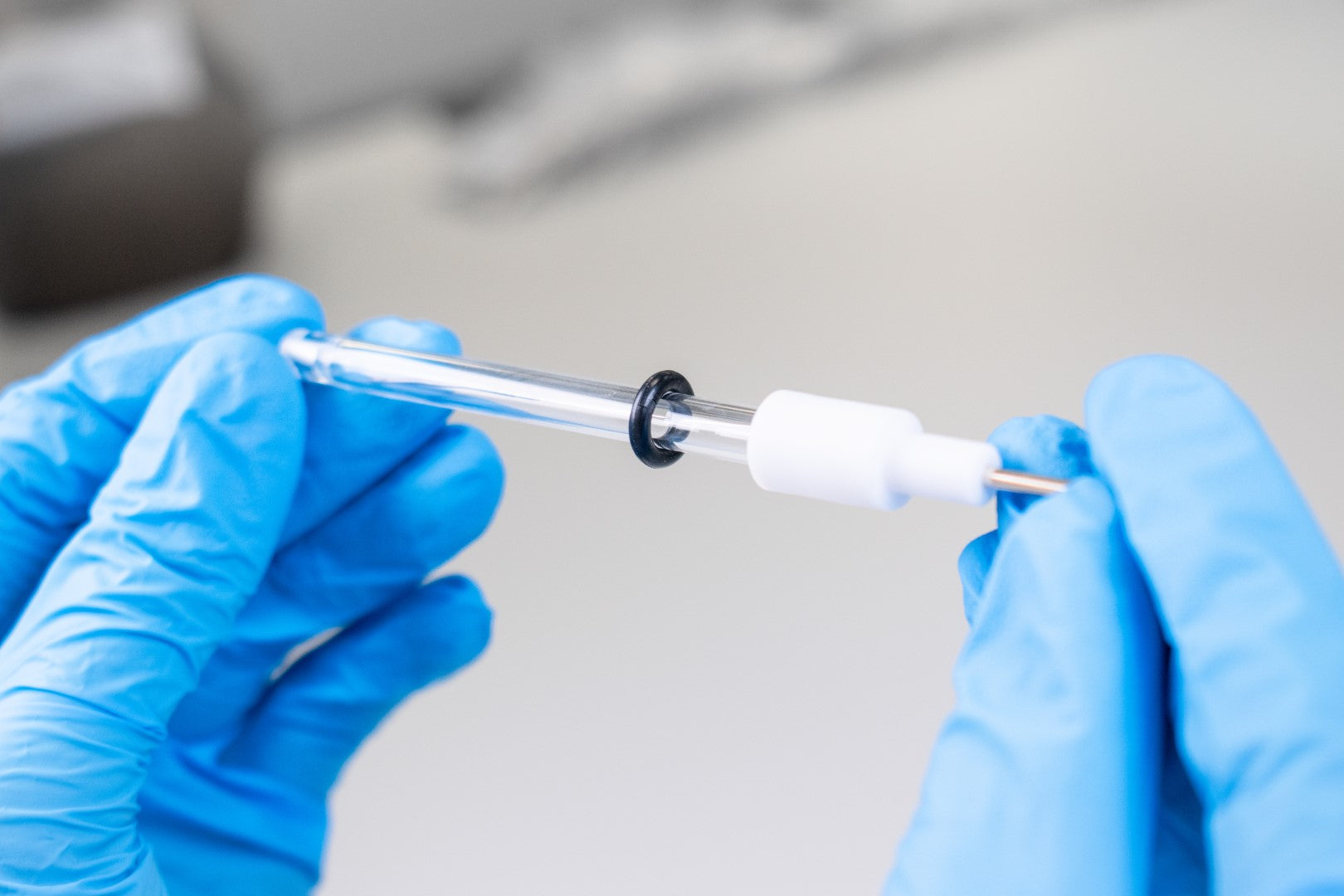
The reference electrode has a white cap which holds the silver or silver/silver chloride wire. The cap is detachable with a gentle force. Hold the glass tube and twist the white cap gently and pull it away from the glass tube. The glass tube is delicate so do not apply a strong force while holding the glass tube.
Once the cap is removed, use a syringe to inject the electrolyte to about 2/3 of the glass tube volume. We suggest gently flicking your index finger on the glass tube to get rid of any air bubbles in the electrolyte.
Put the white cap back on and rinse the reference electrode with acetonitrile. Wipe the electrode clean, air-dry for a few minutes, and now the electrode is ready to work.
Captions
- Remove the electrode from its casing.
- This video is for the preparation of both Ag/Ag+ and Ag/AgCl reference electrodes.
- Check the electrode for damage.
- Gently remove the white electrode cap which holds the silver or silver chloride wire without applying a strong force to the glass tube.
- Place down the silver or silver chloride wire and glass tube
- Prepare an appropriately sized syringe (we used 2 ml) with the required electrolyte solution.
- Electrolyte Solutions: Ag/Ag+ - 0.01M silver nitrate acetonitrile solution, Ag/AgCl - 3M potassium chloride aqueous solution
- Inject the electrolyte solution to about 2/3 of the glass tube volume.
- Gently flick your index finger on the glass tube to get rid of any air bubbles in the electrolyte.
- Return the silver wire to the glass tube and make sure to secure tightly, being careful not to damage the glass tube.
- Remove the black cap off the base of the electrode.
- Rinse the electrode with distilled water
- Gently wipe the electrode clean and leave to air-dry for a few minutes.
- You reference electrode is now ready to work!
You may notice some leakage from the frit while it reaches its equilibrium (wetting of the frit) to allow ionic transport to the electrode. Once it is stabilized, there should be no further leakage.
If the reference electrode is not in use, then it should be kept wet with the glass frit being immersed in the electrolyte. If the reference is not to be used for a long time, then empty the solution from inside the reference electrode, wash well with distilled water and acetone, dried in air and kept in a dark cool dry place.
Electrodes
Learn More
Contributing Authors
Written by
Application Scientist
Video by
Graphic Designer