What is Graphene?
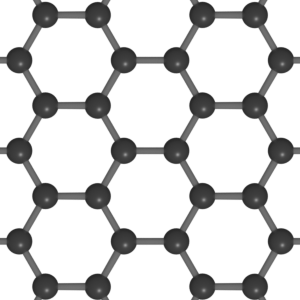
Graphene is a hexagonal lattice of carbon atoms that connect to form a single sheet in 2 dimensions. Each carbon atom is bonded to three other carbon atoms in the x and y planes but nothing in the z plane. Therefore, a graphene sheet is atomically thick as it has the height of just one carbon atom. The thickness of graphene has been measured at ~0.4 - 1.7 nm, which is 250,000 times thinner than printer paper. Theoretically, there is no limit to the size that graphene sheets can become. The largest sheets of graphene have lengths on the centimetre scale which is ten million times larger than its thickness.
Graphene is part of a family of carbon-based (graphitic) materials such as graphite, carbon nanotubes, and fullerenes. They have different structural forms of carbon that share a similar atomic arrangement. Graphite is made up of layers of graphene. There are weak inter-layer interactions that hold graphite together. The interactions are weak enough to allow the layers to slide over each other. This makes it easy to access individual layers of graphene.
The existence of graphene has been well-known for a long time, with TEM images of multi-layer graphene structures being taken as early as the 1940's. However, research into this material only properly began in 2004, when a simple method for isolating single layers of graphene was discovered.
Initial work on graphene showed that its properties vastly exceeded those of the bulk layered graphite from which it was taken. Properties such as the strength showed that a single layer of the material is over 200 times stronger than steel. The mobility of charge carriers like electrons are comparable to that of bulk metals such as copper. Graphene's thermal conductivity is extremely high, allowing heat to pass through it almost without any resistance. Graphene is remarkably transparent despite being a good absorber of light. It absorbs about 2.3% of visible light, a significant amount given that it is only one atom thick.

All of these unique properties mean that graphene could find use in a wide variety of applications including electrochemical capacitor devices, anti-corrosion coatings, composite materials, transparent conducting films, thermal pastes, as well as sensing and biosensing applications.
Graphene

- Versatile
- High Purity
- Low Price
Available from £100
Learn More
References
Wang, C. et al. (2019). Large-Area Synthesis and Growth Mechanism of Graphene by Chemical Vapor Deposition. IntechOpen. DOI: 10.5772/intechopen.79959