2-Bromo-5-dodecylthiophene
CAS Number 153561-74-1
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, Monomers
Another widely used alkylated thiophenes
Offers great solubility, thermal stability, and film morphology in application of OFETs and OPVs
Specifications | MSDS | Literature and Reviews
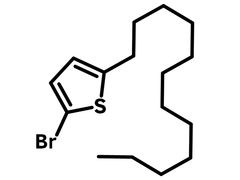
2-Bromo-5-dodecylthiophene (CAS number 153561-74-1), bearing a dodecyl alkyl chain (C12H25) and a bromo function group at both end positions of the thiophene ring, is widely used for the synthesis of semiconducting small molecules, oligomers, and conjugated polymers in the application of organic electronics.
2-Bromo-5-dodecylthiophene is obtained by reacting 5-dodecylthiophene with N-Bromosuccinimide (NBS). The end bromo group provides the functionality for cross coupling or nucleophilic aromatic substitution reactions to offer extended conjugation to the targeted molecules. The long dodecyl alkyl chain offers great solubility thus improves the processability for organic electronics in solutions. Typically, with dodecyl being on the end of the thiophene ring, 2-bromo-5-dodecylthiophene is used as an end-capper especially in the case of OFETs materials.
2-Bromo-5-dodecylthiophene has also been presented in the synthesis of semiconducting calamitic and discotic liquid crystals, owing to the flexible long alkyl chain and the rigid thiophene/phenylene core.
Thiophene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Capped with bromides
for facile coupling reactions
High purity
>98% Purity
General Information
| CAS Number | 153561-74-1 |
| Chemical Formula | C16H27BrS |
| Full Name | 2-Bromo-5-dodecylthiophene |
| Molecular Weight | 311.35 g/mol |
| Synonyms | N/A |
| Classification / Family | Thiophene derivatives, Dyes, Semiconductor synthesis intermediates, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | N/A |
| Boiling Point | Tb = 371.2±22.0 °C at 760 mmHg |
| Appearance | Yellow liquid |
MSDS Documentation
 2-Bromo-5-dodecylthiophene MSDS Sheet
2-Bromo-5-dodecylthiophene MSDS Sheet
Literature and Reviews
-
Asymmetric alkythienyl thienoacenes derived from anthra[2,3-b]thieno[2,3-d]thiophene for solution-processable organic semiconductors, Y. Ogawa et al., ACS Appl. Mater. Interfaces, 9, 9902−9909(2017); DOI: 10.1021/acsami.6b15793.
-
Bent-core mesogens with thiophene units, K. Geese et al., J. Mater. Chem., 20, 9658–9665(2010); DOI: 10.1039/C0JM01919D.
-
Influence of conjugation axis on the optical and electronic properties of aryl-substituted benzobisoxazoles, B. Tlach et al., J. Org. Chem., 78(13), 6570–6581(2013); DOI: 10.1021/jo4007927.
