5-Bromo-3-fluorosalicylaldehyde
CAS Number 251300-28-4
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers
A fluorinated multifunctional building block
Used as a starting material for synthesizing Schiff base for OLED, DSSCs and bioactive compounds
Specifications | MSDS | Literature and Reviews
5-Bromo-3-fluorosalicylaldehyde, CAS number 251300-28-4, is a derivative of salicylaldehyde (2-hydroxybenzaldehyde), bearing functional groups of both bromo- and a fluoro-halogens, a hydroxyl and carboxylic acid around the benzene ring. 5-Bromo-3-fluorosalicylaldehyde is an ideal candidate for synthesizing Schiff base which is a sub-class of imine (ketimines or aldimines). The synthesis of Schiff base starts with nucleophilic addition to the aldehyde with an amine (either aliphatic or aryl) forming a hemiaminal, then followed by dehydration to form the aldimine.
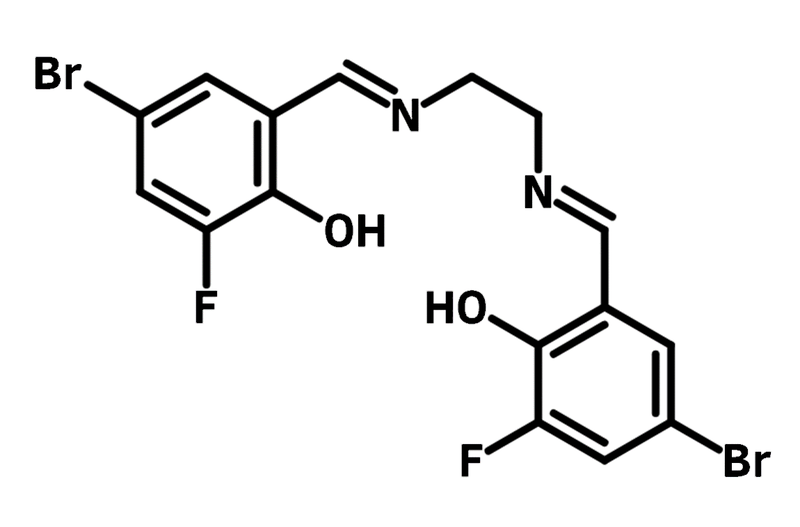
The Schiff bases synthesised from 5-Bromo-3-fluorosalicylaldehyde are bidentate/tetradentate ligands. They can further form coordination complexes that are used as dyes in OLED and dye-sensitized solar cells (DSSCs with power conversion efficiency circa 7.7 %). Such complexes are also investigated in antimicrobial activities against bacteria and fungi.
Multiple functional groups
For facile synthesis
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
Fluorinated salicylaldehyde building block
For OLEDs, dyes, and solar cells
General Information
| CAS Number | 251300-28-4 |
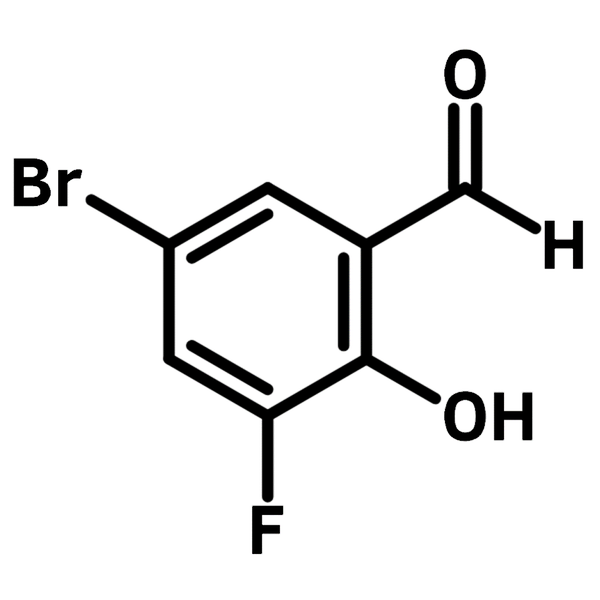
| Chemical Formula | C7H4BrFO2 |
| Full Name | 5-bromo-3-fluoro-2-hydroxybenzaldehyde |
| Molecular Weight | 219.01 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building block, Schiff base, Dyes, Semiconductor synthesis intermediates, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >97% |
| Melting Point | Tm = 112 °C – 116 °C |
| Appearance | Yellow powder |
MSDS Documentation
 5-Bromo-3-fluorosalicylaldehyde MSDS Sheet
5-Bromo-3-fluorosalicylaldehyde MSDS Sheet
Literature and Reviews
-
Schiff bases and their complexes in organic light emitting diode application, S. Kagatikar et al., J. Electron. Mater., 50, 6708–6723(2021); DOI:10.1007/s11664-021-09197-9.
-
Tuning the electronic and charge transport properties of Schiff base compounds by electron donor and/or acceptor groups, A. Irfan et al., Materials, 15, 8590(2022);10.3390/ma15238590.
-
A novel class of Zn(II) Schiff base complexes with aggregation-induced emission enhancement (AIEE) properties: Synthesis, characterization and photophysical/electrochemical properties, Y.-Z. Xie et al., Dyes Pigm., 96, 467-474(2013); DOI:10.1016/j.dyepig.2012.09.020.
