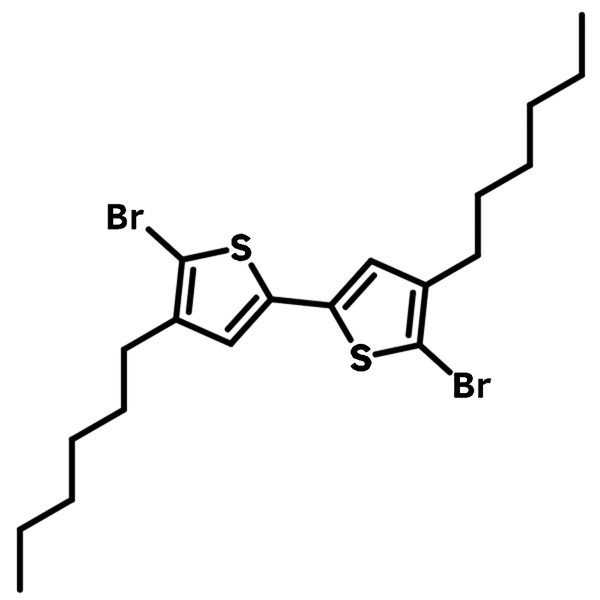
5,5'-Dibromo-4,4'-dihexyl-2,2'-bithiophene
CAS Number 214493-03-5
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, MonomersAn alkylated bithiophene building block with two bromo-functional groups
A bithiophene derivatives for semiconducting oligomer/polymer synthesis in application of OFETs and OSCs
Specifications | MSDS | Literature and Reviews
5,5'-Dibromo-4,4'-dihexyl-2,2'-bithiophene (CAS number 214493-03-5) is a derivative of bithiophene with two hexyl groups at 4,4'-positions and two bromine group at 5,5'-positions, allowing further functionalization as well as gaining excellent solubility. It is facile for synthesising thiophene oligomer/polymer and block copolymer/small molecules with other building blocks such as perylene, triphenylamine and carbazoles.
5,5'-Dibromo-4,4'-dihexyl-2,2'-bithiophene has a well-defined structure with two thiophene units joined at 2,2'-positions and two hexyl groups being away from each other at 4,4'-positions. This is particularly useful when certain desired structure conformation to be achieved or film morphology issues to be addressed, unlike the random coupling reactions between mono-thiophenes.
Bithiophene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Capped with bromides
for facile coupling reactions
High purity
>98% Purity
General Information
| CAS Number | 214493-03-5 |
| Chemical Formula | C20H28Br2S2 |
| Full Name | 5,5'-dibromo-4,4'-dihexyl-2,2'-bithiophene |
| Molecular Weight | 492.37 g/mol |
| Synonyms | 2-bromo-5-(5-bromo-4-hexylthiophen-2-yl)-3-hexylthiophene |
| Classification / Family | thiophene derivatives, Semiconductor synthesis intermediates, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >98% (1H NMR) |
| Boiling Point | Tb > 468.8±40.0 °C at 760 mmHg |
| Appearance | Yellow liquid |
| Relative density | 1.4±0.1 g/cm3 |
MSDS Documentation
 5,5'-Dibromo-4,4'-dihexyl-2,2'-bithiophene MSDS Sheet
5,5'-Dibromo-4,4'-dihexyl-2,2'-bithiophene MSDS Sheet
Literature and Reviews
- Thiophene in conducting polymers: synthesis of poly(thiophene)s and other conjugated polymers containing thiophenes, for application in polymer solar cell, F. Livi et al., Top. Heterocycl. Chem., 39, 203–226(2015); DOI: 10.1007/7081_2014_128.
- Nanoscale correlation between exciton dissociation and carrier transport in silole-containing cyclopentadithiophene-based bulk heterojunction films, J.-H. Huang et al., J. Phys. Chem. C, 115, 2398–2405(2011); DOI: 10.1021/jp1090894.
- Polymer for electronics and spintronics, P. Bujak et al., Chem. Soc. Rev., 42, 8895–8999(2013); DOI: 10.1039/c3cs60257e.
