3DPA2FBN
CAS Number 1403850-00-9
High Purity Sublimed Materials, Materials, OLED Materials, Semiconducting Molecules, TADF MaterialsCyanoarene Photocatalyst and Photosensitizer
TADF photosensitizer, available in sublimed (≥99%) and unsublimed (≥98%) grade
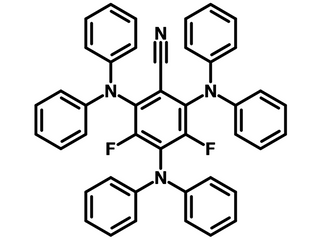
3DPA2FBN, bearing three diphenylamine (DPA) at meta-positions to each other and two fluorine atoms in between the diphenylamine substituents, is a cyanoarene TADF photosensitizer. 3DPA2FBN has been commonly used as a donor–acceptor (D–A) type photocatalyst in visible light induced single electron transfer (SET) mechanism to facilitate chemical or biochemical reactions.
Umpolung carboxylations with CO2 for α-ketoamides and α-ketoesters by employing 3DPA2FBN as the photozensitizer show high selectivity, broad substrate scope, and good functional group tolerance in mild reaction conditions. Facile derivations of products to bioactive compounds were accessed, including oxypheonium, mepenzolate bromide, benactyzine, and tiotropium.
Both 3DPA2FBN and 3DPAFIPN have three electron-donating DPA moieties attached to the benzene ring, showing similar oxidation potentials and hence similar HOMO energy levels. The difference between them is that 3DPAFIPN has two electron-accepting cyano and one fluoro substituents while 3DPA2FBN has one cyano and two fluoro substituents in between the three diphenylamine groups. As cyano group is more electron-withdrawing than fluorine atom, 3DPAFIPN is relatively a stronger ground state oxidant than 3DPA2FBN.
General Information
| CAS number | 1403850-00-9 |
|---|---|
| Full name | 2,4,6-Tris(diphenylamino)-3,5-difluorobenzonitrile |
| Chemical formula | C43H30F2N4 |
| Molecular weight | 640.72 g/mol |
| Photocatalyst Activation | 455 nm |
| Fluorescence | λem 491 nm in acetonitrile |
| HOMO/LUMO | E1/2 = - 1.92 V (vs. SCE) [4] |
| Classification / Family | Triphenylamine, Cyanoarene, TADF materials, Photosensitizers, Photocatalysts |
Product Details
| Purity | Unsublimed >98.0% (1H NMR), Sublimed > 99% |
|---|---|
| Melting point |
280-281 °C |
| Appearance | Yellow powder/crystals |
Chemical Structure
MSDS Documentation
Literature and Reviews
- E. Speckmeier et al. (2018); A Toolbox Approach To Construct Broadly Applicable Metal-Free Catalysts for Photoredox Chemistry: Deliberate Tuning of Redox Potentials and Importance of Halogens in Donor–Acceptor Cyanoarenes, J. Am. Chem. Soc., 140 (45), 15353–15365; DOI: 10.1021/jacs.8b08933.
- K. Donabauer et al. (2020); Photocatalytic Reductive Radical-Polar Crossover for a Base-Free Corey–Seebach Reaction, Chem. Eur. J., 26, 12945–12950; DOI: 10.1002/chem.202003000.
- M. Bryden et al. (2021); Organic thermally activated delayed fluorescence (TADF) compounds used in photocatalysis, Chem. Soc. Rev.,
50, 7587; DOI: 10.1039/d1cs00198a.

 3DPA2FBN MSDS sheet
3DPA2FBN MSDS sheet