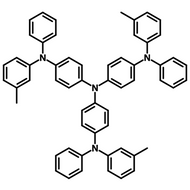
Semiconducting Molecules
Semiconducting molecules are organic compounds which exhibit semiconducting properties. Semiconducting molecules are composed of carbon-based organic compounds, unlike traditional semiconductors like silicon which are inorganic materials.
Semiconducting molecules include hole and electron transport layer, host, and first generation fluorescent, second generation phosphorescent, third generation thermally activated delayed fluorescent (TADF) and fourth generation multi-resonance thermally activated delayed fluorescent (MR-TADF) materials in application of organic light emitting diodes (OLEDs), organic field-effect transistors (OFETs) and photovoltaic devices.
Features and Properties
- Tuneable conductivity. Semiconducting molecules can conduct electricity. Their properties can be tuned by modify the chemical structure, allowing precise control over their conductivity.
- Low cost-manufacturing. Low cost, solution-based processing methods can be used leading to reduced device manufacturing costs. Semiconducting molecules are also generally cheaper to produce than inorganic semiconductors.
- Flexibility. Organic molecules are more flexible than inorganic materials, enabling the fabrication of flexible or stretchable devices.
Explore our small-molecule semiconductors for use as emitters and interlayers in TADF-based OLEDs, active materials in OFETs, and as interface materials to perovskites. Maximize your device efficiency by fabricating and testing new devices in a glove box environment.
Jump to: Semiconducting Molecules by Role | Browse all Semiconducting Molecules | Resources and Support
Semiconducting Molecules by Role
Charge Transport Layer Materials
Explore our range of charge transport layer semiconducting molecules.
Browse Semiconducting Molecules
Related categories: Transport layer materials, Dopant materials, Host materials, TADF materials, Small molecule OPV donors
Filter by charge transport:
Filter by photoluminescence:
Filter by emission colour:
Filter by purification technique:
Page 1 of 7
Resources and Support
Fluorescence and phosphorescence are types of photoluminescence. Photoluminescence refers to radiative emissions where the absorbance of a photon is followed by the emission of a lower energy photon. The main empirical difference between fluorescence and phosphorescence is the time in between absorbance and the emission of photons. Fluorescence is where a material absorbs a photon, and almost immediately emits a lower energy photon.
Read more... Understanding HOMO and LUMO
Understanding HOMO and LUMO
HOMO (Highest Occupied Molecular Orbital) and LUMO (Lowest Unoccupied Molecular Orbital) are fundamental concepts in molecular orbital theory. HOMO and LUMO are known as the "frontier molecular orbitals" because they are the highest occupied and lowest unoccupied molecular orbitals, respectively.
Read more... Organic Light Emitting Diodes
Organic Light Emitting Diodes
Organic light emitting diodes are thin film devices that convert electrical energy into visible light. In OLED devices, electrons and holes are injected into the organic medium and recombine radiatively via electroluminescence (EL). The colour of the light emitted is dependent on the molecular structure of the organic material. This can be easily customized to make a wide range of wavelengths thanks to the endless configurations of organic molecules.
Read more... OPV and OLED Fabrication Guide
OPV and OLED Fabrication Guide
Ossila’s pre-patterned ITO substrates are used for a wide variety of teaching and research devices (both organic and inorganic) where a high-quality ITO surface is required.
Read more...