2D Materials
2D materials can broadly be grouped by their properties and potential applications. They are materials which consist of atomically thin layers that are weakly bonded to one another by van de Waals forces.
From 2D semiconductors in optoelectronics and nanotechnology to flexible electrodes, energy storage and anti-corrosion coatings, our 2D materials – including graphene and molybdenum disulfide – offer a broad range of applications. Explore potential uses in groundbreaking biomedical applications such as tissue engineering, sensors, and drug deliveries, as well as advanced aerospace applications.
Interest in 2D materials has increased dramatically since the successful mechanical exfoliation of graphene in 2004 by Novoselov and Geim. 2D materials can be created from a bulk material via several different methods, the simplest of which is mechanical exfoliation. Also known as the Scotch-tape method, the weak weakly van der Waals forces that bond the single-atom-thick layers in the material together can be overcome with a piece of sticky tape. More sophisticated methods like chemical vapor deposition can be used for larger and more uniform 2D flakes.
Our collection offers high quality, low cost 2D materials in crystal, powder, or film form.
Jump to: 2D Materials by Family | Popular 2D Materials | Browse all 2D Materials | Resources and Support
2D Materials by Family
Popular 2D Materials
Browse 2D Materials
Related categories: low dimensional materials, nanodots and quantum dots, carbon nanotubes
Filter by function:
Filter by family:
Filter by metal:
Filter by form:
Page 1 of 3
Resources and Support
 Introduction to 2D Materials
Introduction to 2D Materials
The foundation of technology is the understanding of material systems. Specific material properties are required depending on the application.
Read more... Viscoelastic Transfer of 2D Material Using PDMS
Viscoelastic Transfer of 2D Material Using PDMS
Viscoelastic transfer using polydimethylsiloxane (PDMS) stamps is one of the methods used for the deterministic placement of 2D materials and the fabrication of van der Waals heterostructures.
Read more... Molybdenum Disulfide (MoS2): Theory & Applications
Molybdenum Disulfide (MoS2): Theory & Applications
Molybdenum disulfide belongs to a class of materials called 'transition metal dichalcogenides'. Materials in this class have the chemical formula MX2, where M is a transition metal atom and X is a chalcogen.
Read more... Reducing Graphene Oxide to Graphene Using Environmentally Safe Materials
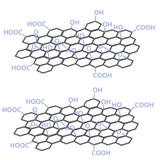
Reducing Graphene Oxide to Graphene Using Environmentally Safe Materials
Graphene has many potential electronic, optoelectronic and biological uses. However, graphene itself is non-soluble, and this makes it very difficult to deposit from solution.
Read more...