Graphene vs Graphite

The graphene materials, graphene and graphite, are both composed entirely of carbon atoms. Graphene and graphite are Graphene is a single layer of carbon atoms arranged in a hexagonal pattern, like a sheet of paper. Graphite, on the other hand, is made up of many layers of graphene stacked on top of each other, like a stack of paper. The graphene layers in graphite are held together by weak forces, allowing them to slide over each other easily.
Graphene has generated a lot of interest recently. This surge in interest particularly increased in 2010 when Andre Geim and Kostya Novoselov were awarded the Nobel Prize in Physics "for ground-breaking experiments regarding the two-dimensional material graphene". Graphene has some exceptional properties which distinguishes it from its bulk form, graphite. Having said this, graphite is useful in different ways due to the ability of it's layers to slight over each other.
| Properties | Graphene | Graphite |
|---|---|---|
| Dimensionality | 2D | 3D |
| Thickness |
~ 1 nm One atom thick Not visible by eye |
> 5 nm Many atoms thick Can be visible by eye |
| Surface Area |
Extremely high specific surface area ~2600 m2/g |
Low surface area compared to graphene 1 m2/g |
| Appearance | Transparent and colorless | Opaque and black/gray |
| Strength |
Strongest material known to exist Tensile strength = 130 GPa Elastic Modulus = 1.1 TPa |
Brittle Tensile strength = 4.8 GPa |
| Bonding |
Each carbon participates in three σ bonds (C-C) and a π bond (hybridized sp2 bonding) Bond lengths = 0.142 nm |
Each carbon participates in three σ bonds (C-C) and a π bond (hybridized sp2 bonding) Bond lengths = 0.142 nm Interlayer van der Waals interactions. Interlayer spacing = 0.335 nm |
| Conductivity |
Exceptionally electrically and thermally conductive Thermal = 5 x 103 W/mK Electron Conductivity = 106 S/m Resistance = 31 Ω/sq Charge mobility = (2 x 105 cm2/V.s) |
Relatively good conductivity but not as good as graphene Thermal = 24 W/mK Resistivity = 0.006 Ω/cm Conductivity = 3.3x102 – 3x105 S/m |
| Stability |
Chemically reactive edges and surface The surface can be functionalized with other elements |
Relatively resistant to chemical reactions |
| Lubricating | Coefficient of Friction = 0.1 | |
| Applications | Electronics, energy storage, sensors | Pencils, lubricants, batteries |
Graphene vs Graphite Structure
Within both materials, each carbon atom is bonded to three other carbon atoms, forming a hexagonal lattice. These carbon-carbon bonds are σ bonds. Carbon atoms have four valence electrons available for bonding. Since only three electrons are used to form σ bonds with other carbon atoms, one electron remains. Each carbon atom in this structure is described as being sp2 hybridized. The remaining electron resides in a pz orbital and can participate in forming a π bond. This network of π bonds is responsible for graphene's remarkable conductivity and other unique properties.
In 2D graphene, the spare electron in the p orbital is free to move throughout the π bonded network. This delocalized electron movement contributes to graphene's exceptional electrical conductivity and other distinctive properties.
In 3D graphite, the movement these spare electrons causes areas of difference in charge across the graphene layers. The slight positive and slight negative areas means van der Waals interactions hold the layers of graphene together.
Graphene vs Graphite Properties
Graphene
Graphene stands out as having exceptional properties such has ultrahigh surface area and excellent thermal and electrical conductivity. These properties arise from it's 2D nature and π bonding network.
Graphene's ultrahigh surface area (~2600 m2/g ) means it would take only 2.7 g to cover a football field. It is therefore useful in surface active applications, including energy storage, sensors and water purification.
Graphene's electrical conductivity as a result of the delocalized π electrons is many orders of magnitude larger than silicon (1.56×102 S/m) which is commonly used in electronic devices. It's thermal conductivity is also outstanding making it suitable for applications in thermal management, such as heat sinks and electronic cooling systems.
Graphite
Graphite is most commonly used in pencils but it can be used for much more. Due to the weak van der Waals interactions between the layers within graphite, it has excellent lubricating properties. It has been used as a dry lubricant and in lubricating greases.
Graphite maintains a significant amount of the thermal and electronic conductive properties that graphene has. It is far more accessible than 2D graphene which requires more intensive processing to access. It's layered honeycomb structure allows for efficient intercalation and deintercalation of lithium ions, contributing to high energy density and long lithium-ion battery life. Graphite is also good in heat management applications. Graphite's inert nature means it is corrosion resistant which is highly desired in electronic applications to maintain performance.
Graphene Materials
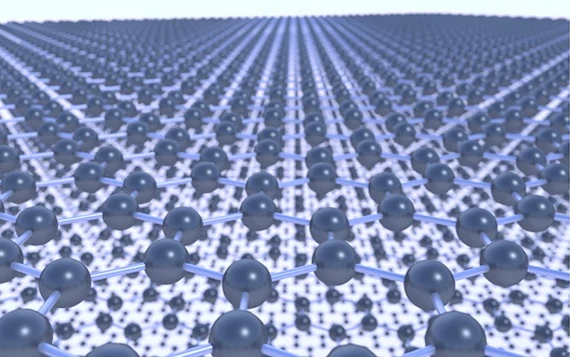
Learn More
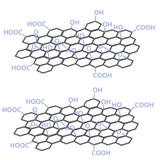 What is Graphene Oxide?
What is Graphene Oxide?
Graphene oxide (GO) is a two-dimensional material with oxygen-functionalized surfaces, derived from graphite.
Read more... Safely Reducing Graphene Oxide
Safely Reducing Graphene Oxide
The conversion of graphene oxide back to graphene is therefore of huge interest to both the scientific and industrial community.
Read more...
References
- Graphene, related two-dimensional crystals, and hybrid systems for..., Bonaccorso, F. et al., Science (2015)
- Graphite, Carbon, C - MatWeb
- Measurement of the Elastic Properties and Intrinsic Strength..., Lee, C. et al., Science (2008)
- Graphene synthesis, characterization and its applications: A review., Mbayachi, V. B. et al., Results Chem. (2021)
- Table of Electrical Resistivity and Conductivity