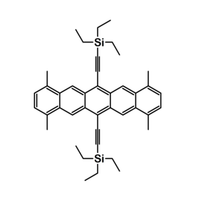
TMTES-Pentacene
Materials, OLED Materials, Semiconducting MoleculesTMTES-Pentacene, an ideal small-molecule semiconductor for device fabrication
Available online for priority dispatch
1,4,8,11-tetramethyl-6,13-triethylsilylethynyl pentacene also known as TMTES-Pentacene. It is a soluble derivative of Pentacene [1], achieving mobilities of up to 4.34 cm2/Vs. TMTES-Pentacene is an ideal small-molecule semiconductor for both device fabrication and investigating the charge transport of solution-processed crystal semiconductor [4]. Mobilities of 3.5 cm2/Vs have been measured when spin coated using CYTOP© as the gate dielectric [2], and mobilities of up to 4.34 cm2/Vs have been achieved when used with high-permittivity polymer binders [3].
General Information
| Full name | 1,4,8,11-tetramethyl-6,13-triethylsilylethynyl pentacene |
|---|---|
| Synonyms | TMTES-pentacene |
| CAS number | n/a |
| Molecular formula | C42H50Si2 |
| Molecular weight | 611.017 g/mol |
| Appearance | Black crystalline solid |
| Solvents | Anisole, butylbenzene, chlorobenzene, chloroform, dichlorobenzene, tetrahydrofuran, toluene, xylene |
| Recommended solvent | Toluene |
| Recommended concentration | 10 mg/ml (drop cast / spin cast) |
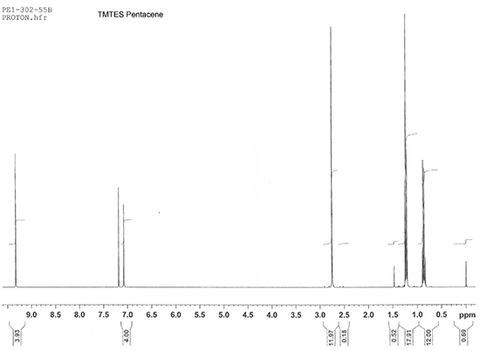
Characterisation
References
(Please note that Ossila has no formal connection to any other authors or institutions in these references):
[1] High performance, acene-based organic thin film transistors. Gonzalo R Llorente et al., Chem. Commun., p3059-3061 (2009)
[2] Charge-transport physics of high-mobility molecular semiconductors. H Sirringhaus et al., Status Solidi B., V 9, 1655-1676 (2012)
[3] High Performance Organic Transistors Using Small Molecule Semiconductors and High Permittivity Semiconducting Polymers. Keri L McCall et al., Advanced Functional Materials, V 17, Issue 17, p3421-3434 (2007)
[4] Hall-Effect Measurements Probing the Degree of Charge-Carrier Delocalization in Solution-Processed Crystalline Molecular Semiconductors. Jui-Fen Chang et al., PhysRe Lett, V 107, 066601 (2011)
