Small Self-Assembled Monolayer Molecule for High Efficiency Solar Cell
2BrPTZPA, Hole transport or extraction layer for NFA-polymer solar cells and p-i-n perovskite solar cells, Hole selective contact, (4-(3,7-dibromo-10H-phenothiazin-10-yl)butyl)phosphonic acid
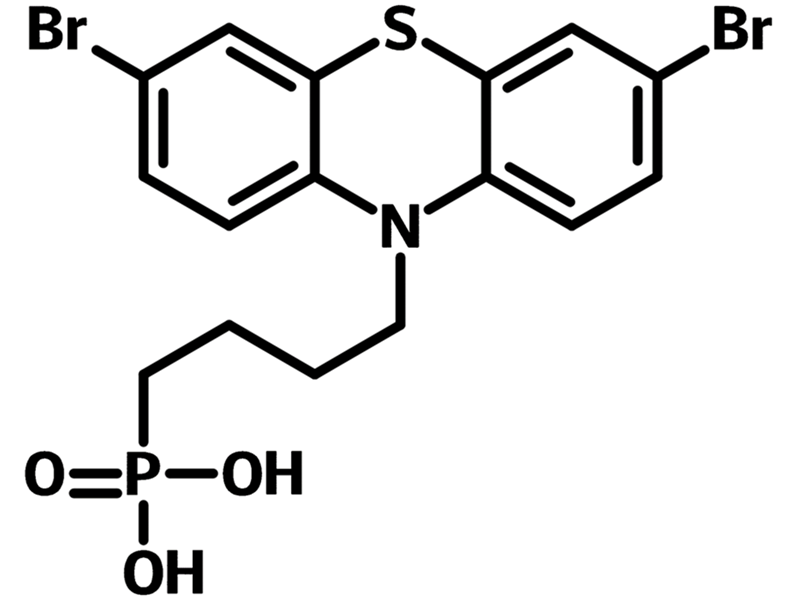
2BrPTZPA is a self-assembled monolayer material that is consisted of a phosphonic acid head anchoring group, a butyl alkyl chain spacer, and a 3,7-dibromo-10H-phenothiazine terminal. 2BrPTZPA has similar structure to Br-2EPT with the only difference between them being the length of the spacer. 2BrPTZPA has a alkyl chain length of four carbons and Br-2EPT has two.
While carbazole derivates have been a popular terminal function groups for the self-assembled monolayer materials for inverted perovskite solar cells, phenothiazine (PTZ) and its derivatives are also electron donating groups which can be considered as doping electron rich sulfur (S) atom in carbazole. SAMs bearing phenothiazine function terminals can effectively modulate their highest occupied molecule orbital (HOMO) levels and facilitate the growth of superior quality perovskite crystals, enabling remarkable continuity in the vertical direction without the presence of discernible grain boundaries. As a result, this gives enhanced hole-extraction in a device, along with the passivation effect of S by coordinating with Pb2+ from perovskites to boost the device efficiency and stability, gaining PCE of 22.06% by engaging 2BrPTZPA as the hole selective contact monolayer between the active perovskite and the ITO anode.
Serving as hole selective contact for organic solar cells and perovskite solar cells, 2BrPTZPA is an alternative to PEDOT:PSS with superior performance with the convenience of solution deposition at low concentration, i.e. 1 mM.
Solution Processing Procedure
Typical processing solvents: THF, ethanol, methanol, IPA, DMF
Typical concentration: 1 mM (0.493 mg/ml) or 1.0 mg/ml (1 mg 2BrPTZPA is dissolved in 1 ml THF)
Typical processing procedure: 30 uL of 2BrPTZPA in THF solution (1.0 mg/ml) is deposited onto the centre of the substrate surface and spin-coated for 30 s at the speed of 3000 rpm. The coated film on the substrate is then annealed for 10 min at 100 ℃ (DOI: 10.1039/D2NR05677A).
General Information
| CAS Number | n.a. |
|---|---|
| Chemical Formula | C16H16Br2NO3PS |
| Molecular Weight | 493.15 g/mol |
| Absorption* | λmax (319 nm in THF, 324 nm in film) |
| Fluorescence | λem (n.a.) |
| HOMO/LUMO | HOMO = 5.51 eV, LUMO = 2.52 eV |
| Synonyms | Br-4BPT, (4-(3,7-dibromo-10H-phenothiazin-10-yl)butyl)phosphonic acid |
| Classification or Family | 10H-phenothiazine derivatives, Self-assembly monolayers, Hole transport layer, Hole extraction layer, p-i-n Perovskite solar cells, Organic photovoltaics |
Product Details
| Purity | > 98% (HPLC) |
|---|---|
| Melting Point | Td = 234 °C |
| Appearance | Blue powder/crystals |
Chemical Structure
MSDS Documentation
Literature and Reviews
- P. Ganesan et al. (2024); Structural divergence of molecular hole selective materials for viable p-i-n perovskite photovoltaics: a comprehensive review, J. Mater. Chem. A, 12, 12983-13058; DOI: 10.1039/D4TA01453G.
- Z. Li et al. (2023); Simple and robust phenoxazine phosphonic acid molecules as self-assembled hole selective contacts for high-performance inverted perovskite solar cells, Nanoscale, 15, 1676-1686; DOI: 10.1039/D2NR05677A.
- Z. Yi et al. (2023); Self‐assembled monolayers (SAMs) in inverted perovskite solar cells and their tandem photovoltaics application, Interdisciplinary Mater., 3, 203–244; DOI: 10.1002/idm2.12145.
Licensed by Helmholtz-Zentrum Berlin für Materialien und Energie GmbH in Germany and Kaunas University of Technology in Lithuania.

 2BrPTZPA MSDS Sheet
2BrPTZPA MSDS Sheet