Applications of Fullerene and Fullerene Derivatives

Fullerene and its derivatives are used in chemical, electronic, medicinal, and biological sciences due to their unique physical and chemical properties.
Fullerene, also known as C60 or buckminsterfullerene, is a type of carbon allotrope that has properties that can be tuned depending on what it will be used for. Similar molecules, like fullerene C70, have different amounts of carbon atoms. Fullerene derivatives, such as PCBM, have different chemical functional groups attached to the hollow fullerene structures.
Fullerenes are good electron acceptors which makes it useful in electronic applications such as solar cells which harness the sun's energy. It can undergo radical quenching reactions which are helpful in medicinal and biological applications. Fullerene can bond to transition metals, which is useful in the catalysis of reactions. Its spherical shape results in unique properties that can be exploited in biomedicine and as a lubricant.
Fullerene in Electronics
Fullerenes are often used in electronics as semiconductors. Semiconductors conduct energy from an external source. Fullerene’s low lying LUMO and ability to transport electrons well makes it a good n-type semiconductor. An n type semiconductor uses electrons as the charge carriers responsible for conduction, as opposed to p-type semiconductors where holes (space left by electrons) are responsible for conduction. Fullerenes play an important role in electronic device research, since devices made with fullerenes are thinner, more lightweight, and more flexible than silicon-based devices.
Solar Cells
Fullerenes can be used as electron transport or electron accepting layers, depending on the type of solar cell being manufactured. Fullerenes are commonly used in bulk heterojunction solar cells as electron acceptor materials, alongside other types of organic solar cell. Bulk heterojunction solar cells have acceptor and donor layers that are inter-dispersed so there is as much contact between the two layers as possible.
Fullerene derivative PCBM has been a key n-type semiconductor in the production of organic solar cells. Other common n-type semiconductors used in solar cell fabrication include C60, C70, and PC70BM. Perovskite solar cells also exploit the characteristics of fullerenes, and are used as electron transport layers. Fullerenes are good electron transport layers because of their electron accepting nature.
Batteries
Fullerenes can be used in batteries as dopants or sustainable cathodes. The abundance of carbon makes them attractive in the development of new, more sustainable batteries. Adding fullerene to anodes can result in an increase in cell voltages. The ability to tweak the characteristics of fullerene makes them appealing to scientists, as different functional groups or elements can be added to the structure of fullerene. Fullerene has been used in lithium ion batteries to improve the sustainability of the cathodes in some batteries. Fullerene has also been used in the electrodes of rechargeable batteries.
Fullerenes in Chemistry
Fullerene is an electrophile, an electron loving molecule. This means it can undergo several different chemical reactions:
- Addition reactions
- Condensation reactions
- Radical reactions
- Reactions with transition metals
It has a low lying lowest unfilled molecular orbital (LUMO), meaning it can promote an electron to the excited state easily. This means that it is an effective semiconductor and photosensitiser.
Free Radical Reactions
The electrophilicity of fullerene means it is very reactive towards free radicals. Free radicals are a species that have one unpaired electron in their outer shell. This means fullerenes can function as an antioxidant, ‘sucking up’ free radicals from their surrounding environment. Free radicals are unfavourable as they are in an unstable electronic configuration. When one free radical reacts, this reactions forms a new free radical. This results in a chain reaction. Fullerene is able to use its electrophilicity to stop this chain reaction. Radicals can react with cells, damaging the cells by degrading the molecules that they are built from. The use of fullerene in skincare can prevent cell damage.
Fullerene also acts as a photosensitizer and can be used in photodynamic therapy. A photosensitiser is a molecule that absorbs a photon then transfers that energy to another molecule. Photosensitizers work by absorbing light and undergoing intersystem crossing to be in the triplet excited state. This excited state then reacts with triplet oxygen to form singlet oxygen and radical oxygen species. The radical species can then degrade the surrounding cells, which is useful in cancer treatments or in antimicrobial treatments.
Lubricants
Due to its spherical shape, fullerene has been exploited as a solid lubricant and as an additive to existing lubricants. It is commonly used when the conditions are not appropriate for a liquid lubricant, for example at high temperatures. The small spheres can provide lubrication by rolling over one another and rolling between the two solids.
Catalysis
Fullerenes can react with electron rich transition metals to form transition metal complexes (Fullerene-TM). Metals will typically have two bonds to the fullerene molecule. These two bonds are usually between two six membered rings. Fullerene-TM complexes are often useful in catalysis.
There are three ways fullerene can be used in catalysis:
- As a catalyst itself
- As a ligand for a metal in homogeneous catalysis
- As a supporting molecule in heterogeneous catalysis
Fullerene-TM can be used as a catalyst to break down H2O into H2 and O2, a reaction that is important in the synthesis of hydrogen fuel and in industrial chemical manufacturing. The use of fullerene as a catalyst for this reaction can reduce the amount of rare metal used in catalysis. The electronic structure of fullerene allows H2O to adsorb to the surface of the catalyst more effectively.
Fullerenes in Medicine and Biology
Fullerenes are used in biological and medicinal applications due to their ability to form free radical oxygen species on contact with light, form singlet oxygen, and quench free radicals. Due to the aqueous conditions usually required for medicine and biology water-soluble fullerenes such as fullerenol and fullerene C60 malonic acid.
Drug Delivery
Fullerene is used in drug delivery systems due to its ability to interact well with cells and also pass through cell membranes. Fullerene and its derivatives can deliver small molecules and DNA directly to a cell. Fullerene based drug delivery systems can designed to be site specific, and can be designed to be made water soluble using hydrophilic functional groups.
Cancer Treatments and Anti-Microbial Applications
There are two functions fullerene can take on in cancer treatments, the first using its free radical forming abilities and the second utilising fullerene as a ‘radical sponge’.
Fullerene can decrease the toxicity of medicines to healthy cells. It can but put into the body to quench cell damaging free radical species that form because of medical treatments such as radiation. Fullerene can remain in a tumour and function as a ‘free radical sponge’, as well as being able to inhibit tumour growth.
Photodynamic therapy uses reactive oxygen species produced by the photosensitiser to fight cancer cells in the body. It can provide precise treatment to cells resulting in less damage of surrounding cells as opposed to other cancer treatments.
Antimicrobial photodynamic inactivation (aPDI) uses a similar mechanism to photodynamic therapy, using reactive oxygen species to tackle microbes.
Skincare
Fullerene is used as an antioxidant ingredient to prevent aging and skin cancer. It scavenges radicals produced by UV light on the skin to neutralise them and prevent them from harming the skin, acting as a free radical sponge.
PCBM
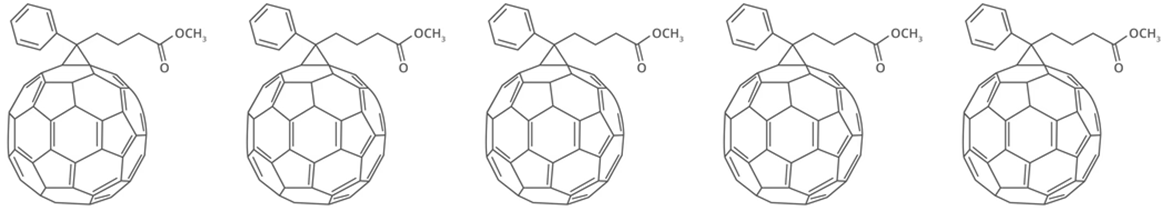
Learn More
 Properties of Fullerene
Properties of Fullerene
Fullerene C60 exhibits unique structural, chemical, electronic, and thermal properties. This is due to its highly symmetrical icosahedral structure that contains a mixture of single and double bonds. All fullerenes have partial electron sharing across the molecule and a high affinity for electrons.
Read more... Water-Soluble Fullerenes: Preparation and Applications
Water-Soluble Fullerenes: Preparation and Applications
Water-soluble fullerenes, such as fullerenol C60, are either chemically modified, polymer grafted, or encapsulated in a hydrophillic agent. Fullerenes can then disperse or solubilize in aqueous media.
Read more...
References
- Electron and hole transport in solution-processed fullerenes, Gert-Jan A. H. Wetzelaer and Paul W. M. Blom, Journal of Materials Chemistry C (2021)
- Efficiency of bulk-heterojunction organic solar cells, M.C. Scharber, N.S. Sariciftci, Progress in Polymer Science ( 2013)
- Probing Mechanistic Insights into Highly Efficient Lithium Storage..., Haifa Qiu, Jing Wan, Junxian Zhang, Xin Wang, Nianji Zhang, Rouxi Chen, Yu Xia, Li Huang, Hsing-Lin Wang, Advanced Science (2021)
- Efficiency of bulk-heterojunction organic solar cells, M.C. Scharber, N.S. Sariciftci, Progress in Polymer Science (2013)
- Medicinal applications of fullerenes, Rania Bakry, Rainer M Vallant, Muhammad Najam-ul-Haq, Matthias Rainer, Zoltan Szabo, Christian W Huck, and Günther K Bonn, Int J Nanomedicine (2007)




