Properties of Fullerene
Fullerene C60 (also known as carbon-60) exhibits unique structural, chemical, electronic, and thermal properties. This is due to its highly symmetrical icosahedral structure that contains a mixture of single and double bonds. All fullerenes have partial electron sharing across the molecule and a high affinity for electrons. This makes fullerenes great at accepting electrons which is attractive for use in solar cells which harness the sun's energy.
Structural Properties of Fullerene
In C60 fullerene, there are twelve pentagonal rings in between the twenty hexagonal benzene rings. Each carbon atom has two single bonds along adjacent sides of a pentagon and one double bond between two adjoining hexagons. The curvature of the C60 surface, due to the strain from the pentagons, causes the planar orbitals to hybridize. Within fullerene there is some sp3 character in the classic sp2 bonding found in benzene-based structures. This prevents the complete delocalisation of the π-electrons. Therefore, the double bonds become susceptible to addition reactions. Which does not happen in classical aromatic compounds.
Are Fullerenes Aromatic?
Fullerene is generally not considered to be aromatic, despite having a conjugated π-electron system with partial delocalization. The mixture of sp2 and sp3 bonds with different bond lengths means electrons are not fully delocalized. This gives fullerenes non-aromatic characteristics such as chemical reactivity.
| Aromatic Properties | Non-Aromatic Properties |
|---|---|
|
Evidence that fullerenes experience substantial ring currents An external electromagnetic field causes electrons to circulate round the hexagonal rings. Considerable stability Delocalization of electrons allows the charge density to be spread out across multiple atoms, reducing the electron-electron repulsion which stabilizes the fullerene molecule. |
Chemical reactivity Fullerenes are very reactive and easily undergo a large variety of chemical transformations that, unlike most aromatic compounds, are in most cases addition reactions to the conjugated π system. Different bond lengths Shorter for double bonds and longer for single (should be more uniform if aromatic). Partial delocalization The mixture of sp2 and sp3 bond character leads to partial delocalization of electrons. |
High Symmetry of Fullerene
Fullerenes are highly symmetrical, especially C60 fullerene which has icosahedral symmetry (Ih). They have 2-fold, 3-fold and 5-fold symmetry. This is visualized below. The pentagons direct this symmetry. You can see the lines of symmetry go directly through the points of the pentagonal carbons.
Electronic Properties of Fullerene
Electronic Structure of Fullerene
Since all the bonding (valence) requirements of the carbon atoms are satisfied, C60 is a semiconductor with a relatively small gap between HOMO and LUMO. The semiconducting behavior is stabilized by the distortions in the intramolecular bond lengths. Depending on the conditions of the fullerene the gap changes in size. Environment also effects whether the gap it is direct or indirect.
Optical Effects
The absorption of photons that cause an electron within fullerene to transition to a different energy level is symmetry-forbidden near the band gap. This is due to the high symmetry of fullerene. As a result, fullerene has very low absorption strengths making it relatively transparent to light at energies just above its band gap. This means fullerene isn't very efficient at absorbing solar radiation making it less attractive to applications trying to maximize harnessing the suns energy. However, fullerene’s transparency at certain wavelengths can be advantageous in specific organic electronic applications. For example, in transparent electronics or in layers of devices where light needs to pass through.
Tuning the Electronic Structure of Fullerene
Doping of fullerene and its derivatives can be carried out to further tune the band gap. When C60 is doped with alkali metals, the added electrons occupy the LUMO, effectively turning C60 into a metallic conductor or even a superconductor at low temperatures. This transformation is a result of the increased delocalization of π-electrons, which enables the free movement of charge carriers.
For a fullerene anion (C60⁻), an additional electron is added to the system, typically occupying the LUMO. The presence of this extra electron modifies the electronic structure slightly, leading to new electronic states that are closer in energy to the ground state—these are known as low-lying excited states. Low lying excited states of the fullerene anion promote fast charge separation. This is because there are intermediate energy levels that electrons can immediately access.
Electron Acceptor Properties
Fullerene has an electron affinity of 2.6 to 2.8 eV which indicates it can easily accept electrons. Therefore fullerenes are classed as electron acceptors. This is a really attractive property to those that are looking to harness the suns energy to produce electricity (photovoltaics). Fullerenes are usually partnered with electron donor materials that absorb the suns light and donate electrons as a result. The high electron affinity of fullerene ensures that electron transfer is energetically favorable. This is crucial for efficient charge separation and the generation of electrical current in solar cells.
Unfortunately, fullerenes can undergo degradation on exposure to light (photo-oxidation) which reduces their ability to act as an electron acceptor. The fullerene oxide has a deeper LUMO level which can act as an electron trap, reducing conductivity.
Why is Fullerene a Poor Electrical Conductor?
The charge mobility of fullerene C60 and its derivatives is generally on the order of 10−4 to 10−3 cm2 (V s)−1. This is a low electrical conductivity especially compared to graphene (2 x 105 cm2/V.s). This is due to its partially delocalized electrons, large band gap, and the symmetry-forbidden transitions that prevent easy electron excitation. Fullerene's conductivity is still high enough for some organic electronic devices. Especially when its other properties, like electron acceptance, are so great.
Chemical Properties of Fullerene
Chemical Reactivity of Fullerene
The chemical reactivity of fullerene and its derivatives comes from their mixed bonding characteristics and is often induced by light. Fullerenes can undergo lots of types of reactions including:

- Reduction
- Nucleophilic Addition
- Cycloaddition
- Hydrogenation
- Radical Addition
- Transition Metal Complex Formation
- Oxidation
- Reaction with Electrophiles
Surface Modification of Fullerene
The surface modification of fullerene C60 has given rise to numerous derivatives. Functional groups have been added to fullerene which introduce different chemistry to its surface. Fullerenes not only have the features of fullerene C60, but also show improved physiochemical properties such as:
- excellent conductivity
- appropriate wettability
- ease of processing
- specific surface functionality for a given application
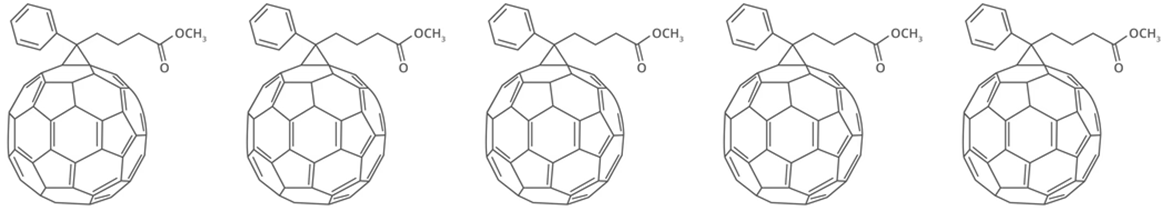
The most famous example of this is PCBM which stands for [6,6] phenyl-C61-butyric acid methyl ester. The phenyl and butyric acid methyl ester functional groups have given this fullerene interesting and attractive features. They phenyl group can undergo π–π stacking either with other PCBM molecules or other components in a device. The butyric acid methyl ester acts as a solvent or processing additive. It helps with uniform blending and optimizes the morphology when PCBM is interacting within a complex system of materials.
Free Radical Sponge
Due to its high electrophilicity, fullerene is highly reactive toward free radicals. Free radicals are species with an unpaired electron in their outer shell, making them unstable and prone to initiating chain reactions. Fullerenes act as potent antioxidants by utilizing their electrophilic nature to "absorb" free radicals from the surrounding environment, effectively neutralizing them. This prevents the free radicals from continuing the chain reaction that would otherwise generate more radicals. If left unchecked, these radicals can damage cells by breaking down essential molecules. Fullerenes leverage their electrophilicity to disrupt this harmful process and protect cellular structures.
Solubility
Fullerenes have aromatic character so are hydrophobic and therefore are insoluble in water. They are soluble in organic solvents, especially those that are functionalised like PCBM. The increased solubility of PCBM because of its phenyl and ester functional groups means it can be more easily solution processed. This is helpful when making solar devices where film morphology is important. The more smoothly the fullerene can be processed, with fewer defects, the more efficient the device is likely to be.
Thermal Properties of Fullerene
Effect of Changing Temperature on Fullerene
Each type of fullerene experiences different changes in state (phase transitions) when exposed to different temperatures. Different amounts of heat energy are required to heat one unit of mass by one unit of temperature (specific heat). Generally, the impact of changing temperature on the specific heat of C60 is comparable to graphite at temperatures over 250K (-23.15 °C or -9.67 °F). This means there are a similar number of ways to store energy (degree of freedom) in both C60 and graphite.
Thermal Conductivity
Fullerenes have low thermal conductivities, especially when compared to other carbon based materials like graphene (5 x 103 W m−1 K−1) :
- C60 fullerene - 0.2 W m−1 K−1
- PCBM - ~0.075 W m−1 K−1
There are fewer charge carriers to carry thermal energy in fullerenes so atomic vibrations contribute most to thermal conductivity. Fullerenes have more than three orders of magnitude lower thermal conductivity than graphite. This is because there are more defects in fullerenes which scatter the charge carriers and reduce their ability to carry heat. As well as this, the phonons within fullerenes are low-frequency and therefore don’t contribute to thermal conductivity.
PCBM
Learn More
 How are Fullerenes Made?
How are Fullerenes Made?
Today, fullerenes are made using three main methods; Huffman-Krätschmer, combustion and microwave. Chemical synthesis techniques, such as laser irradiation and pyrolysis, offer exciting possibilities for producing fullerene derivatives that were previously not accessible.
Read more... What are Fullerenes?
What are Fullerenes?
Fullerenes are an allotrope of carbon and are known for their hollow, cage-like structures. They are composed entirely of carbon atoms that form closed, convex polyhedra. The atoms are bonded together to form hexagonal and pentagonal rings, like the pattern found on a soccer ball or geodesic dome.
Read more...
References
- Fullerenes, Dresselhaus, M. S., Journal of Materials Research (2011)
- Fundamental Properties and Applications of Fullerene and Carbon..., Scharff, P., Frontiers of Multifunctional Nanosystems (2002)
- Fullerenes, Dresselhaus, M. S., Journal of Materials Research (1993)
- The significance of fullerene electron acceptors in organic..., Materials Science and Technology (2016)
- Fullerenes: the stars of photovoltaics, Collavini, S., Sustainable Energy Fuels (2018)
Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer