2-Chloro-5-fluoropyrimidine
CAS Number 62802-42-0
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, Monomers
A fluorinated pyrimidine building block
Applied as a synthesis intermediate for APIs and OLEDs
Specifications | MSDS | Literature and Reviews
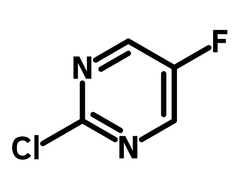
5-Fluoro-2-chloropyrimidine (CAS number 62802-42-0) is derived from pyrimidine, an aromatic heterocyclic 6-membered ring with two nitrogen atoms at positions 1 and 3. 5-Fluoro-2-chloropyrimidine also has a fluorine and a chlorine substituents at the para-position, making it useful for synthesizing linear molecules. With two nitrogens in the ring, 5-fluoro-2-chloropyrimidine is π electron deficient which facilitates nucleophilic aromatic substitution. 2-Chloro-5-fluoropyrimidine is often used to synthesize active pharmaceutical ingredients (APIs), such as antibiotics.
5-Fluoro-2-chloropyrimidine is also used to synthesize ligands for iridium complexes. The iridium complexes show great external quantum efficiency exceeding 29.5%.
Multiple functional groups
For facile synthesis
Fluorinated pyrimidine building block
For drug discovery, organometallic ligands and semiconducting materials
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 62802-42-0 |
| Chemical Formula | C4H2ClFN2 |
| Full Name | 2-Chloro-5-fluoropyrimidine |
| Molecular Weight | 132.52 g/mol |
| Synonyms | 5-Fluoro-2-chloropyrimidine |
| Classification / Family | Fluorinated building block, Heterocyclic building block, APIs, OLEDs |
Chemical Structure
Product Details
| Purity | 98% |
| Boiling Point | Tb = 172 – 174 °C at 760 mmHg |
| Relative Density | 1.073 g/mL at 25 °C |
| Appearance | Colourless liquid |
MSDS Documentation
 2-Chloro-5-fluoropyrimidine MSDS Sheet
2-Chloro-5-fluoropyrimidine MSDS Sheet
Literature and Reviews
-
Discovery of SYD5115, a novel orally active small molecule TSH-R antagonist, W. Karstens et al., Bioorg. Med. Chem., 84, 117258(2023); DOI: 10.1016/j.bmc.2023.117258.
-
Novel pyrimidine-piperazine hybrids as potential antimicrobial agents: in-vitro antimicrobial and in-silico studies, S. Rejinthala et al., Results in Chemistry, 5, 100951(2023); DOI: 10.1016/j.rechem.2023.100951 .
-
Synthesis, evaluation, and mechanism study of new tepotinib derivatives as antiproliferative agents, N.-N. Zhang et al., Molecules, 24, 1173(2019); DOI: 10.3390/molecules24061173.
