3-Fluoro-p-anisidine
CAS Number 366-99-4
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers
A fluorinated p-anisidine building block
Used as a precursor for heterocycles in application of APIs
Specifications | MSDS | Literature and Reviews
3-Fluoro-p-anisidine (CAS number 366-99-4), also referred to as 3-fluoro-4-methoxyaniline, is an aniline with a fluoride and a methoxy substituted at 3- and 4-positions. 3-Fluoro-p-anisidine is commonly used as a precursor for synthesising quinoline derivatives through Combes quinoline synthesis with 1,3-diketones. The as synthesised quinoline derivatives are employed as active pharmaceutical ingredients and dyes. 3-Fluoro-p-anisidine is readily attached to molecular scaffolds through nucleophilic substitution.
The primary amine in 3-fluoro-p-anisidine can be used to prepare azides for copper-catalysed azide-alkyne cycloaddition (click reaction).
Multiple functional groups
For facile synthesis
Fluorinated aniline building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 366-99-4 |
| Chemical Formula | C7H8FNO |
| Full Name | 3-Fluoro-4-methoxyaniline |
| Molecular Weight | 141.15 g/mol |
| Synonyms | 4-Amino-2-fluoroanisole, 3-Fluoro-4-methoxyaniline, 4-Amino-2-fluoro-1-methoxybenzene |
| Classification / Family | Fluorinated building blocks, Aniline building blocks, APIs, Heterocycles |
Chemical Structure
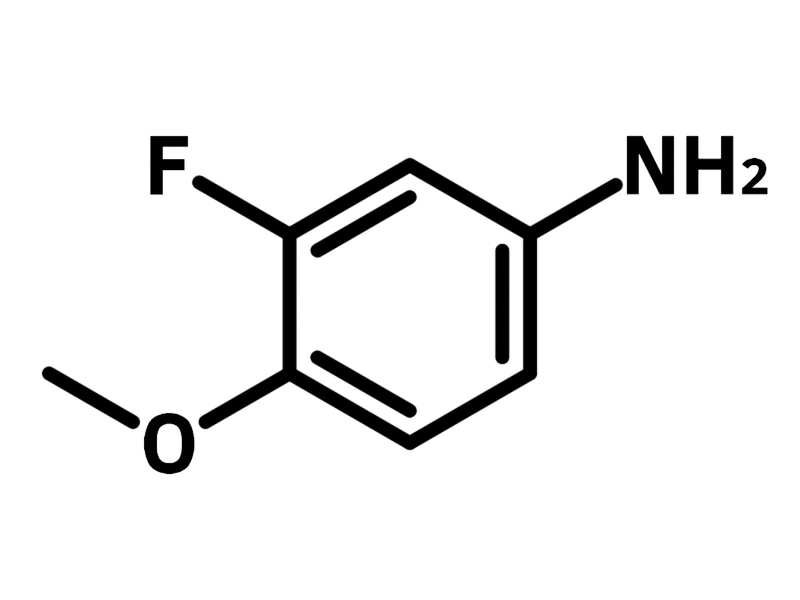
Product Details
| Purity | 97% |
| Melting Point | Tm = 81 °C – 83 °C |
| Appearance | Brown powder |
MSDS Documentation
 3-Fluoro-p-anisidine MSDS Sheet
3-Fluoro-p-anisidine MSDS Sheet
Literature and Reviews
- 7-Alkoxy-4-phenylamino-3-quinolinecar-bonitriles as dual inhibitors of Src and Abl kinases, D. Boschelli et al., J. Med. Chem., 47(7), 1599–1601(2004); DOI: 10.1021/jm0499458.
- CuI-mediated synthesis of 1-aryl-5,6,7-trimethoxybenzimidazoles as potent antitubulin agents, C.-M. Peng et al., RSC Adv., 13, 13169(2023); DOI: 10.1039/d3ra01927f.
- Fluoride-containing podophyllum derivatives exhibit antitumor activities through enhancing mitochondrial apoptosis pathway by increasing the expression of caspase-9 in HeLa cells, W. Zhao et al., Sci. Rep., 5, 17175(2015); DOI: 10.1038/srep17175.
