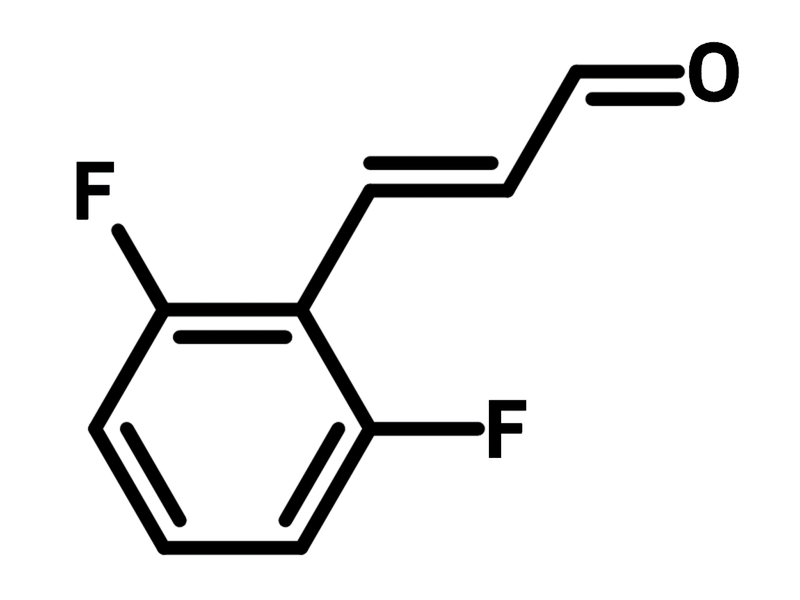
2,6-Difluorocinnamic aldehyde
CAS Number 117338-43-9
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers
A fluorinated cinnamaldehyde building block
Used as a synthesis intermediate for APIs and macromolecules
Specifications | MSDS | Literature and Reviews
2,6-Difluorocinnamic aldehyde (CAS number 117338-43-9) is a cinnamaldehyde derivative with fluorine substituents at 2- and 6-position. 2,6-Difluorocinnamic aldehyde includes a propenal functional group, allowing it to engage in conjugative addition with Gillman reagents and direct addition onto the aldehyde using Grignard reagents. 2,6-Difluorocinnamic aldehyde reacts with methylbenzimidazole through nucleophilic substitution for antimicrobials, yielding antimicrobial compounds with demonstrated potency of 32 µg/mL.
When subjected to an oxidative condensation reaction catalysed by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), 2,6-difluorocinnamic aldehyde and dipyrromethene yield hexaphyrin molecules. Notably, the alkene moiety present in 2,6-difluorocinnamic aldehyde proves advantageous for ruthenium-catalysed C-H activations.
Multiple functional groups
For facile synthesis
Fluorinated cinnamic aldehyde building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 117338-43-9 |
| Chemical Formula | C9H6F2O |
| Full Name | 2,6-Difluorocinnamic aldehyde |
| Molecular Weight | 168.14 g/mol |
| Synonyms | 3-(2,6-Difluorophenyl)acrylaldehyde, 3-(2,6-Difluorophenyl)prop-2-enal |
| Classification / Family | Fluorinated building blocks, Aldehyde building blocks, APIs, Macromolecules |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | N/A |
| Appearance | White crystals |
MSDS Documentation
 2,6-Difluorocinnamic aldehyde MSDS Sheet
2,6-Difluorocinnamic aldehyde MSDS Sheet
Literature and Reviews
- Cinnamaldehyde derivatives act as antimicrobial agents against Acinetobacter baumannii through the inhibition of cell division, W. Chai et al., Front. Microbiol., 13, 967949(2022); DOI: 10.3389/fmicb.2022.967949.
- Corroles and hexaphyrins: synthesis and application in cancer photodynamic therapy, S. Lopes et al., Molecules, 25, 3450(2020); DOI: 10.3390/molecules25153450.
- Inhibition of spontaneous mutagenesis by vanillin and cinnamaldehyde in Escherichia coli: dependence on recombinational repair, D. Shaughnessy et al., Mutat. Res., 602(1-2), 54–64(2006); DOI: 10.1016/j.mrfmmm.2006.08.006.
