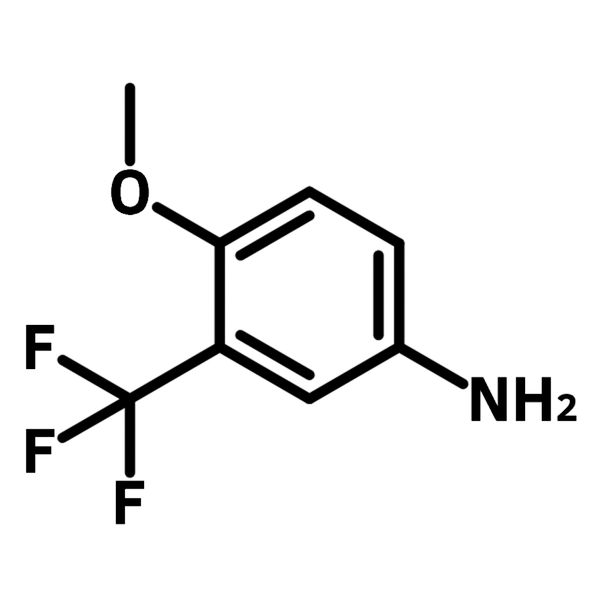
4-Methoxy-3-(trifluoromethyl)aniline
CAS Number 393-15-7
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers
A fluorinated anisidine building block
A synthetic substrate in organic synthesis and a precursor for bicyclic heterocycles
Specifications | MSDS | Literature and Reviews
4-Methoxy-3-(trifluoromethyl)aniline (CAS number 393-15-7) is a derivative of anisidine, with a trifluoromethyl substituent introduced at 3-position. 4-Methoxy-3-(trifluoromethyl)aniline is also referred to as anisole, due to the presence of a methoxybenzene moiety. 4-Methoxy-3-(trifluoromethyl)aniline is commonly utilised as a substrate in organic synthesis for studying amine/aniline-related reactions.
4-Methoxy-3-(trifluoromethyl)aniline is also used for synthesising substituted bicyclic heterocycles, including quinolines, benzotriazoles and benzimidazoles analogues. These applications are particularly relevant to the development of active pharmaceutical ingredients (APIs), showing strong antitumor and antiviral activities.
Multiple functional groups
For facile synthesis
Fluorinated aniline building block
for drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 393-15-7 |
| Chemical Formula | C8H8F3NO |
| Full Name | 4-Methoxy-3-(trifluoromethyl)aniline |
| Molecular Weight | 191.15 g/mol |
| Synonyms | 3-(Trifluoromethyl)-p-anisidine, 4-Amino-2-(trifluoromethyl)anisole, 4-Methoxy-3-(trifluoromethyl)benzenamine, 5-Amino-2-methoxybenzotrifluoride |
| Classification / Family | Fluorinated building blocks, Aniline building blocks, Anisidine building blocks, Anisole building blocks, Organic synthesis, Heterocycles |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 58 °C – 60 °C |
| Appearance | Pale pink powder/crystals |
MSDS Documentation
 4-Methoxy-3-(trifluoromethyl)aniline MSDS Sheet
4-Methoxy-3-(trifluoromethyl)aniline MSDS Sheet
Literature and Reviews
- A general method for N-methylation of amines and nitro compounds with dimethylsulfoxide, X. Jiang et al., Chem. Eur. J., 20, 58–63(2014); DOI: 10.1002/chem.201303802.
- Trisubstituted imidazole synthesis: a review, N. Rani et al., Mini-Rev. Org. Chem., 12(1), 34–65(2015); DOI: 10.2174/1570193X11666141028235010.
- Synthesis and leishmanicidal activity of molecular hybrids 1,2,3-trazole-chalcones, S. Rodríguez-Gutiérrez et al., Chem. Proc., 3, 55(2021); DOI: 10.3390/ecsoc-24-08356.
