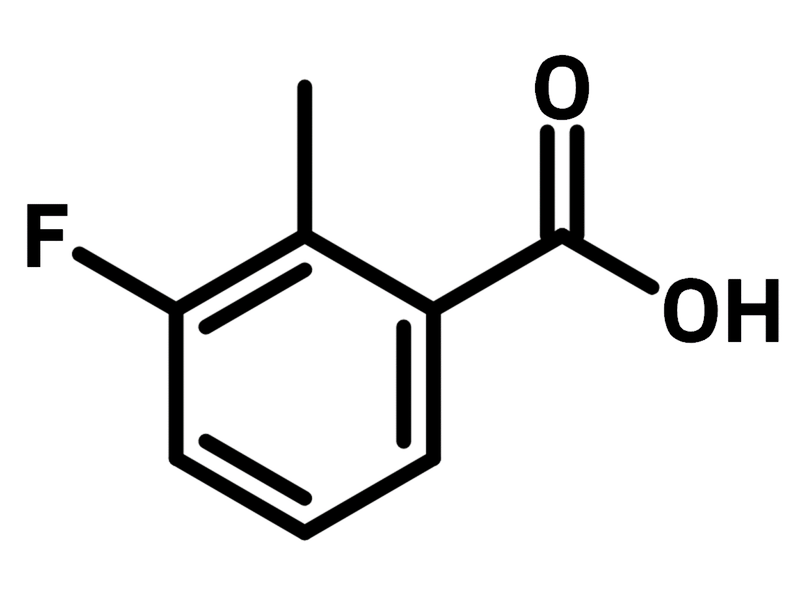
3-Fluoro-2-methylbenzoic acid
CAS Number 699-90-1
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated benzoic acid building block
Used as a synthesis intermediate for APIs and liquid crystals
Specifications | MSDS | Literature and Reviews
3-Fluoro-2-methylbenzoic acid (CAS number 699-90-1) is derived from methylbenzoic acid, also known as ortho-toluic acid. Acetoxy-methylbenzoic anhydride is synthesised from 3-fluoro-2-methylbenzoic acid, demonstrating antibiotic activity with minimum inhibitory concentration (MIC) of 500–1000 μg/mL. Diarylmethanes, which are novel building blocks for sodium-glucose transporter 2 (SGLT2) inhibitors can be obtained from 3-fluoro-2-methylbenzoic acid through Friedel-Crafts acylation, followed by a reduction reaction.
The positioning of the carboxylic acid and fluoride at the meta-position renders 3-fluoro-2-methylbenzoic acid a structural scaffold for bent-core liquid crystals.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
For drug discovery, liquid crystals and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 699-90-1 |
| Chemical Formula | C8H7FO2 |
| Full Name | 3-Fluoro-2-methylbenzoic acid |
| Molecular Weight | 154.14 g/mol |
| Synonyms | 3-Fluoro-o-toluic acid |
| Classification / Family | Fluorinated building blocks, Benzoic acid derivatives, APIs, Liquid crystals |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 158 °C – 160 °C |
| Appearance | White powder |
MSDS Documentation
 3-Fluoro-2-methylbenzoic acid MSDS Sheet
3-Fluoro-2-methylbenzoic acid MSDS Sheet
Literature and Reviews
- Regioselective nitration of 3-fluoro-2-substituted benzoic acids, K. Hurth et al., Tetrahedron Lett., 56(22), 2860–2862(2015); DOI: 10.1016/j.tetlet.2015.04.057.
- Synthesis, spectroscopic characterization, single-crystal structure, Hirshfeld surface analysis, and antimicrobial studies of 3-acetoxy-2-methylbenzoic anhydride, Ş. Çakmak et al., ACS Omega, 7, 17192–17201(2022); DOI: 10.1021/acsomega.2c00879.
- New synthesis of diarylmethanes, key building block for SGLT2 inhibitors, M. Seki et al., ACS Omega, 8, 17288–17295(2023); DOI: 10.1021/acsomega.3c01972.
