Measuring BODIPY Fluorescence with UV Vis Spectroscopy

BODIPY is an organic fluorophore with impressive fluorescent quantum yield, small stokes shift and impressive chemical and photostability. These are often used in biological labelling and as an organic fluorescent dye.
Absorbance and fluorescence spectra for the molecular dye BODIPY-Br (a bromine-substituted boron dipyrromethene derivative) are shown below. These spectra of BODIPY fluorescence were measured using the Ossila USB Spectrometer. Here, we see that optical spectroscopy can be incredibly useful tool for identify and explain the behaviour and properties of small molecules at varying concentrations.
To fabricate the samples, we dissolved BODIPY-Br at varying concentrations in a solution of polystyrene (a transparent polymer) in the solvent toluene. We then coated this onto quartz-coated glass substrates using an Ossila Spin Coater to form 180 nm thin films.
As expected, the absorbance of the films increases as the relative dye concentration is increased. This is because the more absorbent material in the film, the more light is absorbed.

Fluorescence Spectra
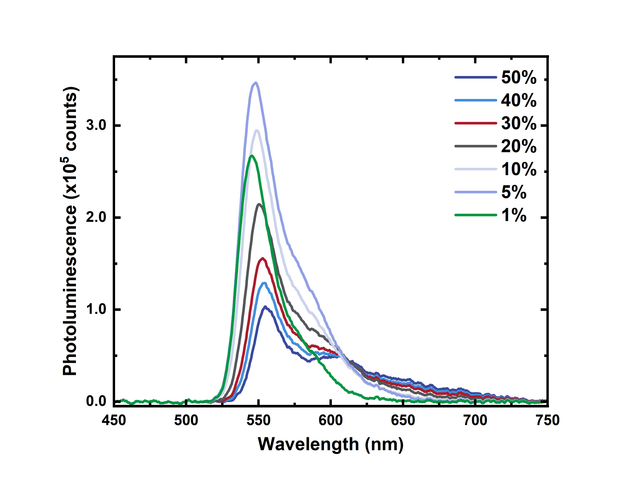
Perhaps unexpectedly, the peak fluorescence intensity of the films does not increase linearly with dye concentration. In fact, the 50% film has the lowest peak intensity, while the 10% film has the greatest.
This reduction in fluorescence intensity at higher concentrations is due to intermolecular interactions caused by the aggregation of the BODIPY-Br molecules. At low concentrations, the fluorescent molecules are spatially separated, and no intermolecular interactions are possible. As the relative concentration of the molecules is increased, the spacing between adjacent molecules is reduced and intermolecular interactions become significant. This reduces the total fluorescence from the sample.
Normalised Fluorescence Spectra
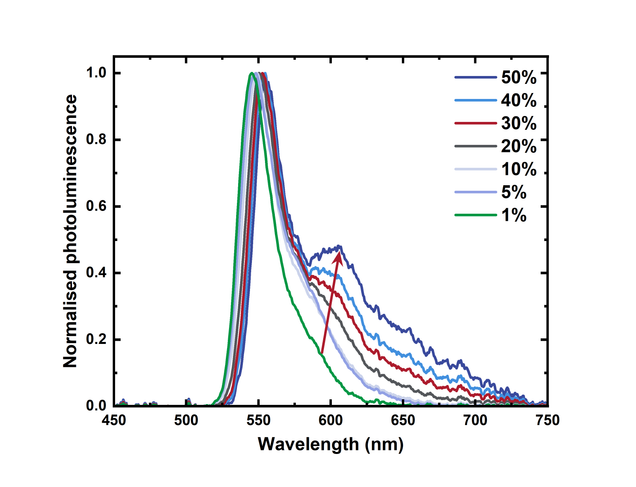
In addition to the reduction in the fluorescence intensity, the spectral shape also changes. The most obvious change is shown in the figure below, which shows the normalised fluorescence spectra. As the concentration of BODIPY-Br increases, the shoulder around 600 nm emerges and increases in intensity. This is also an effect of aggregation and in the case of BODIPY-Br is due to the formation of excimers.
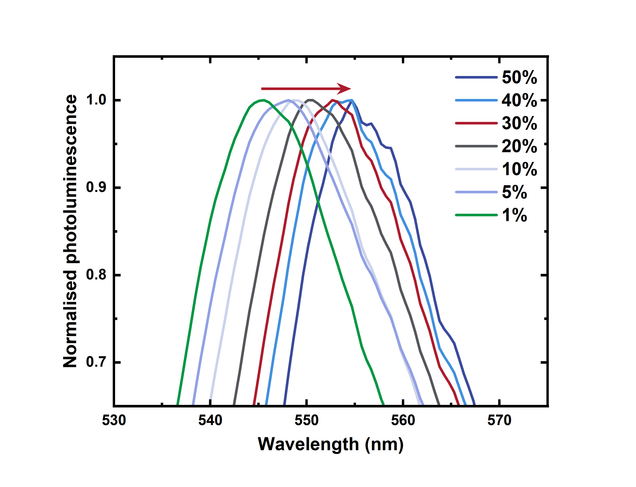
Zooming into the main peak shows how the position of the peak redshifts with increasing concentration. This is further evidence of aggregation resulting in excimer formation.
USB Spectrometer

Learn More
 Optical Fiber Spectroscopy
Optical Fiber Spectroscopy
Optical fibers (or fiber optic cables) are cables which transmit light efficiently along an extremely thin glass (silica) or plastic fiber. Light travels down the cable due to total internal reflection. There is relatively low loss of signal over large distances at specific wavelengths. Optical fibers are often used in telecommunications or data communication. However, they can also be useful in spectroscopy as they can transfer light efficiently between modular spectroscopy elements with little attenuation.
Read more... Exciplex and Excimer: Formation and Emission
Exciplex and Excimer: Formation and Emission
An exciplex (or excited complex) is a complex formed from two different molecules, where one molecule is in an excited state (donor) and the other is in the ground state (acceptor). Both are typically conjugated semiconducting molecules. This short-lived complex will occur when the molecules are in close proximity to one another, and it results in a lower energy than if the two existed separately in their excited and ground states.
Read more...