Defining Moisture Content in a Glove Box

Controlling the moisture content present in a glove box is vital. First, it helps maintain a stable environment for air-sensitive experiments. Second, it prevents moisture-sensitive materials from degrading in the workspace. Lastly, it can improve the accuracy and reliability of your results.
There are multiple ways of defining how much moisture is present in the air. Yet, it is difficult for people to quickly switch between these different definitions and values. With clear definitions, guidance on converting values, and a simple conversion table, you will easily be able to work with any of the available definitions to control the moisture content in your glove box environment.
Vapour Pressure
Vapour pressure is a measurement of the pressure exerted by water vapour in a gaseous form.
The water particles in the liquid phase and water phase exert a pressure on each other. If the pressure of the liquid phase is higher than that of the vapour phase, then moisture can evaporate and become part of the gas phase. As more moisture is present in the air the vapour pressure increases until an equilibrium is reached where the pressure exerted by moisture in the vapour phase is equal to that exerted by water in the liquid phase. This is known as the saturation vapour pressure and is the maximum moisture that air can contain before condensation occurs.
Relative Humidity
The most commonly used definition of moisture content is relative humidity. Relative humidity is a relative measurement based upon a scale from 0 to 100 percent. A value of 0 represents air with no moisture content, while 100 represents air that is fully saturated with water.
With relative humidity the saturation vapour pressure is taken as the maximum achievable relative humidity of 100%. By measuring the current water vapour pressure and comparing it to this maximum value, a relative saturation percentage can be calculated. This is shown below where Pw is the vapour pressure or water and Pws is the saturation vapour pressure.
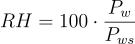
The value of saturation vapour pressure is not constant and is dependent on the temperature of the water at any given time. There are several methods for calculating the saturation vapour pressure. The most commonly used approximation is the Arden Buck equation.
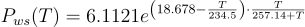
The relative humidity scale, as a function of temperature, is not constant and will vary. Air with the same amount of water in will have a variable relative humidity as the temperature changes over time.
There are various methods for measuring the vapour pressure of air to determine the relative humidity. Digital hygrometers work to measure the electrical properties of air directly or materials in contact with air.
Dew Point
An alternative method for measuring the moisture content of air in your glove box is to measure the dew point. This is the temperature to which air must be cooled for condensation to occur. It can also be defined as the temperature required to reach the saturation vapour pressure for a given level of moisture in the air. By cooling down a sample of air and observing when condensation occurs, you can easily determine the current dew point of any given volume of air.
Knowing the temperature of the air, you can obtain the saturation vapour pressure from the Arden Bucks equation. This will then be equal to the vapour pressure of moisture in the air sample given. Using the temperature of the air, you can calculate the saturation vapour pressure of that air and divide the vapour pressure of the condensed sample to give the relative humidity of the air.
Where a, b, c, and d are constants determined by Arden Buck and are: a = 6.1121 mbar, b = 18.678, c = 234.5 °C, and d = 257.14 °C. Tdp is the dew point temperature and T is the temperature of the air the sample was taken from. Although this is a little more complex, there are advantages of using the dew point. Measurements of dew point are significantly more accurate than those taken using standard hygrometers. The use of cold stages to measure dew points down to below -90 °C can give water vapour pressures as low as 0.1 µBar of pressure.
Parts Per Million (PPM)
By measuring the relative humidity or the dew point of an air sample, the vapour pressure can be determined. From this value, parts per million (ppm) values for moisture content can be obtained. It is important to note that there are two ways of determining the ppm of a sample: the ppm by weight and the ppm by volume fraction.
Where Pw is the water vapour pressure and Ptotal is the absolute pressure.
Where Mw is the molecular mass of water and MD is the molecular mass of the dry gas, which could be air, nitrogen, or argon depending on the atmosphere you are measuring.
Moisture Content Conversions
Different glove boxes define moisture content in different ways. With the standard temperature, pressure, and Arden Buck approximations, we can create a quick conversion table for different relative humidity values, vapour pressure, dew point, and ppm.
| Humidity (%) | Pressure (mbar) | Dew Point (C) | PPM (volume) | PPM (weight) |
| 100 | 24.871695 | 21.00019944 | 25164.145 | 16179.13 |
| 90 | 22.3845255 | 19.29632381 | 22590.8825 | 14524.66 |
| 80 | 19.897356 | 17.41780975 | 20030.5059 | 12878.49 |
| 70 | 17.4101865 | 15.32091908 | 17482.9187 | 11240.53 |
| 60 | 14.923017 | 12.94250482 | 14948.0253 | 9610.737 |
|
50 |
12.4358475 | 10.18625957 | 12425.7311 | 7989.044 |
|
40 |
9.948678 | 6.893913923 | 9915.94228 | 6375.392 |
|
30 |
7.4615085 | 2.775633574 | 7418.56619 | 4769.72 |
|
20 |
4.974339 | -2.799821685 | 4933.51094 | 3171.969 |
|
10 |
2.4871695 | -11.75789851 | 2460.68556 | 1582.082 |
|
9 |
2.23845255 | -13.06037343 | 2214.07219 | 1423.524 |
|
8 |
1.9897356 | -14.49884475 | 1967.58013 | 1265.043 |
|
7 |
1.74101865 | -16.10764253 | 1721.20928 | 1106.641 |
|
6 |
1.4923017 | -17.93641026 | 1474.95957 | 948.3158 |
|
5 |
1.24358475 | -20.06099891 | 1228.8309 | 790.069 |
|
4 |
0.9948678 | -22.60632501 | 982.823172 | 631.8999 |
|
3 |
0.74615085 | -25.8017308 | 736.93631 | 473.8085 |
|
2 |
0.4974339 | -30.14834764 | 491.170219 | 315.7948 |
|
1 |
0.24871695 | -37.18207612 | 245.524812 | 157.8586 |
|
0.5 |
0.124358475 | -43.75722003 | 122.747337 | 78.91962 |
|
0.2 |
0.04974339 | -51.81835899 | 49.0953192 | 31.56552 |
|
0.1 |
0.024871695 | -57.48728061 | 24.547057 | 15.78237 |
|
0.05 |
0.012435848 | -62.82271414 | 12.2733779 | 7.89109 |
|
0.02 |
0.004974339 | -69.41210298 | 4.909315 | 3.156413 |
|
0.01 |
0.00248717 | -74.07811105 | 2.45465147 | 1.578202 |
|
0.005 |
0.001243585 | -78.49406292 | 1.22732423 | 0.7891 |
|
0.002 |
0.000497434 | -83.98086105 | 0.49092933 | 0.31564 |
|
0.001 |
0.000248717 | -87.88835032 | 0.24546461 | 0.15782 |
Glove Box

Learn More
 Glove Box Standard Operating Procedure
Glove Box Standard Operating Procedure
Every glove box requires a good standard operating procedure to ensure proper use. Everyone with access to the glove box should agree to follow this procedure.
Read more... How to Choose a Glove Box
How to Choose a Glove Box
It is important that you choose the most suitable glove box for your samples, experimental needs, budget, and lab environment. .
Read more...