Fluorescence Spectroscopy

Fluorescence spectroscopy is used to measure fluorescence. The technique often used together with absorbance spectroscopy. Fluorescence is a type of photoluminescence where light is quickly reemitted from a material after incident photons are absorbed. This is different to phosphorescence where there is a delay between photon absorption and emission. The term fluorescence is often used interchangeably with photoluminescence.
In fluorescence spectroscopy, a high energy light source, such as a UV light or a laser, is used to excite electrons in your material to a higher energy state. The emitted light from this sample is then measured by a spectrometer, like the Ossila USB Spectrometer, which will detect at what wavelengths light is emitted from your sample. This will give you lots of information about the different energy levels in your sample which can tell you about the optical properties of your substance.
Fluorescence spectroscopy can be used to measure a broad range of organic samples, such as semiconductor devices, optical devices and materials and biological samples.
What is Fluorescence?
Fluorescence spectroscopy can be used to probe energy levels and their associated vibrational energy states within your optical material. We can illustrate what happens during fluorescence using a Jablonski diagram like the one shown here.
Here, we can see that an electron is excited into a higher energy state when a photon is absorbed. The energy of this photon must be greater than difference between the two energy levels. In fluorescence, this electron quickly loses energy through non-radiative radiation – then almost immediately relaxes back to its original energy band, emitting a lower energy photon in the process.
One characteristic of fluorescent transitions, compared to phosphorescent transitions, is that fluorescence only consists of spin-allowed transitions. This means that these emissions happen almost immediately after excitation (on the scale of nanoseconds).
Fluorescence Quenching
Fluorescence quenching refers to processes which can reduce the photoluminescence quantum yield (the amount of fluorescence) by allowing the electron to relax to a lower energy state non-radiatively.
Quenching mechanisms include:
- Vibrational relaxation and internal conversion.
- Intersystem crossing.
- Förster resonance energy transfer.
- Dexter electron transfer.
- Radiative energy transfer.
Measuring Fluorescence
In fluorescence spectroscopy, a beam of light, usually ultraviolet (UV) light, is directed onto the material of interest. This beam of light excites the electrons within the material, causing them to occupy higher energy levels. As the excited electrons return to their normal energy levels, they release energy in the form of light. This emitted light is then collected and analyzed by a spectrometer, which measures the intensity and wavelength of the emitted light. By studying the emitted light, you can gain insights into the composition, structure, and behaviour of the material they are studying.
For fluorescence spectroscopy, you will need:
- An excitation light source
- A spectrometer (or spectrofluorometer)
- For all components to be enclosed in a sealed unit or held in place using an optical breadboard and fixed sample holders
The excitation source that you use must deliver directed light to your sample with a higher energy than the difference between the energy levels you wish to probe. For measuring organic electronics, many of these transitions occur within the visible light region. Therefore, there are multiple light sources that you can use for fluorescence spectroscopy, including diode lasers and UV LED lights. The type of light source required will depend on your sample, the type of fluorescence you wish to conduct and practical matters such as size, price and safety.
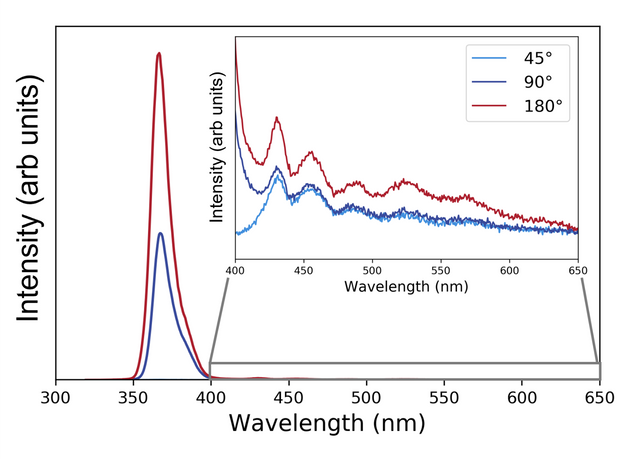
Fluorescence is emitted in all directions equally. Therefore, to do fluorescence spectroscopy, you should conduct measurements at 90° from the excitation beam to avoid interference. You can measure fluorescence straight through a sample, but you will need some kind of optical filter to remove the excitation signal. If you are measuring thin films, you might need to measure at a 45° or 135° angle as you may notice some reflection effects at 90° – especially with specular samples.
Applications of Fluorescence Spectroscopy
Fluorescence spectroscopy is used in many different industries to do chemical and biological analysis of organic materials. Materials that exhibit fluorescent behaviour include:
- Biological samples (chlorophylls, fluorescent proteins)
- Organic dyes, pigments, and phosphors
- Materials used in TADF-OLEDs, OLEDs, and OPVs/PSCs
- Semiconductor devices themselves such as solar cells and organic light emitting diodes
- Quantum dots or nanodots (such as carbon nanodots and perovskite quantum dots)
You can also use fluorescence spectroscopy to track biological processes via Förster resonance energy transfer between fluorophores. Alternatively, fluorescence microscopy can be used to monitor changes in conjugated systems or organic compounds over time or due to changes in environment (solvent, molecular structure, pH, temperature, etc).
Fluorescence microscopy is a reliable and easy way to characterize fluorescence from your optical or biological samples. You can purchase large rigid spectrofluorometers to conduct these experiments, but these are quite expensive and cannot be used for other spectroscopy measurements. The Ossila USB Spectrometer, along with complementary accessories, will supply you with everything you need to begin conducting fluorescence measurements on your samples – whether in solution or on thin films.
USB Spectrometer

Learn More
 What is a Fluorophore?
What is a Fluorophore?
A fluorophore is a chemical compound that is fluorescent, meaning it emits strong glowing colours. There are three key groups of chemical compounds that can fluoresce include quantum dots, conjugated organic molecules and proteins..
Read more... Measuring Thin Film Fluorescence
Measuring Thin Film Fluorescence
Measuring fluorescence (or photoluminescence) with the Ossila Optical Spectrometer will allow you to detect light emission from your sample between 360 nm - 1000 nm. You can use optical spectroscopy as a quick and easy way to characterise fluorescence from your sample. However, you will need an integrating sphere or a more complex system if you want to measure quantitative measurements such as PLQY.
Read more...