Getting Started with the Ossila Potentiostat

What is a potentiostat?
A potentiostat is a control and measurement device designed to output a controlled potential. Unlike other voltage sources, potentiostats contain feedback circuitry between the output and measured potential. This enables them to maintain a set potential through a circuit with varying resistance by increasing or decreasing the output, ensuring that the measured potential remains constant as per Ohm's Law.
Potentiostatic experiments such as cyclic voltammetry use a three electrode system consisting of a working electrode, a counter electrode, and a reference electrode. These are placed in an electrochemical cell containing the electrolyte solution and a reference solution. During a measurement, the potential of the reference electrode remains fixed while the potentiostat varies the potential of the working electrode. The counter electrode completes the circuit and allows current to flow, counteracting the redox events taking place at the working electrode due to electrochemical reactions in the cell, and ensuring that no current passes between the reference and working electrodes.
Getting Started with the Ossila Potentiostat
The Ossila Potentiostat has been designed to make it quick and easy to perform electrochemistry. Purchase the complete package to get everything you need to set up your three electrode system and enjoy a significant discount on the cell and electrodes.
Setting Up the Potentiostat
The Ossila Potentiostat is controlled by the (free to download and use) electrochemistry PC software. To get started, simply set up the physical aspects of your experiment and launch the software.
The experimental set up for cyclic voltammetry and other three electrode electrochemical methods consists of a potentiostat connected to a three electrode electrochemical cell containing the electrolyte solution. Once the electrodes have been placed in the cell, they can be connected to the socket on the front of the Ossila Potentiostat using the supplied cable and crocodile clips. The red clip connects to the working electrode, the black clip connects to the counter electrode, and the blue clip connects to the reference electrode.
Once you have set up your three electrode electrochemical cell and connected it to the potentiostat, taking an electrochemical measurement takes only a few clicks. The potentiostat will be detected automatically on starting the PC software, and from here the electrochemical method can be selected via the tabs at the top of the screen.
We also recommend switching on the potentiostat 30 minutes prior to use. This will allow it to warm up and reach a steady temperature, which will help to ensure a stable measurement.
Performing Cyclic Voltammetry with the Potentiostat
Performing cyclic voltammetry with the Ossila Potentiostat is quick and easy. The Ossila Electrochemistry PC software allows the maximum and minimum potentials to be defined along with the number of cycles, the scan rate, and the current range.
When you are ready to start the scan, click "Start" and watch in real time as the test is performed and a cyclic voltammogram is generated. The system will sweep the potential between the working electrode and reference electrode while measuring the current between the working electrode and counter electrode. This will be repeated for the specified number of cycles. If "Save After Measurement" is turned on, the measurement data and settings will be saved as CSV file once the sweep has finished.
Applications of Cyclic Voltammetry
Cyclic voltammetry reveals a number of important electrochemical properties about the material being investigated, including:
- Redox potentials. The reduction and oxidation potentials of a material describe how readily it gains or loses electrons. Reduction occurs when a chemical gains electron(s), and oxidation is when a chemical loses electron(s). Redox potential is an intrinsic property of materials.
- The reversibility of a reaction. How reversible a given electrochemical reaction is. For a completely reversible reaction, the concentration of oxidised species and reduced species should be in equilibrium.
- Electron transfer kinetics. A quantitative description of electrochemical reversibility; how fast or slow the transfer of electrons is in a reaction. For a reaction to be reversible, electron transfer must be sufficiently fast.
- Energy levels of semiconducting polymers. The energy levels of semiconducting materials. This is particularly useful for photovoltaic applications as it provides an estimate for the energy of the highest occupied (HOMO) and lowest unoccupied molecular orbitals (LUMO).
Applications
The Ossila Potentiostat and Electrochemistry Software make it easy to perform the most commonly used electrochemical methods.
Cyclic voltammetry
Cyclic voltammetry is one of the most useful and commonly used electrochemical techniques. In cyclic voltammetry, a linearly ramping potential is applied between the working and reference electrodes. This potential is cycled such that the ramp is applied in one direction, then in reverse, forming a triangular wave. During the scan, the electrical current is measured between the working and counter electrodes to produce a duck-shaped plot known as a cyclic voltammogram.
Linear sweep voltammetry
Linear sweep voltammetry is an electrochemical method in which the potential is linearly swept in one direction from the lower potential limit to the upper potential limit. Linear sweep voltammetry is useful for irreversible systems and can be used to calculate the peak current, the peak current potential, and the half-peak current potential.
Open circuit potential
Open circuit potential is an electrolytic method that can be used to measure the resting potential of an electrochemical cell between the reference and working electrodes. Systems that are in equilibrium over the timescale of the experiment will have a constant open circuit potential.
Controlled potential electrolysis
Three electrode controlled potential electrolysis is a type of bulk electrolysis (or potentiostatic coulometry) in which the potential between the working and reference electrodes is kept constant while the current through the cell is measured. As the analyte is reduced or oxidised, the measured current will approach zero.

Potentiostat

Learn More
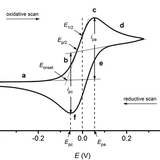 Cyclic Voltammetry Basic Principles, Theory and Setup
Cyclic Voltammetry Basic Principles, Theory and Setup
Cyclic voltammetry is an electrochemical technique used to measure the current response of a redox active solution to a linearly cycled potential sweep.
Read more... Linear Sweep Voltammetry: Introduction and Applications
Linear Sweep Voltammetry: Introduction and Applications
Linear sweep voltammetry (LSV) is a simple electrochemical technique. The method is similar to cyclic voltammetry.
Read more...