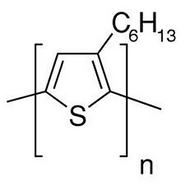
The Impact of Organic Solar Cell Processing Conditions
While a large part of research into the morphology of bulk heterojunction organic solar cells focuses the selection of different components,1 it can also tuned by a host of processing conditions. Morphology can be optimised by modifying the following conditions:2
- Molar mass ratio of the donor:acceptor blend
- Annealing temperature of the active layer
- Cathode buffer layer choice
Key research demonstrating this used an OPV cell with a conventional stack of ITO-coated glass/PEDOT:PSS/active layer/cathode buffer layer/Al, where the active layer was a blend of regioregular P3HT and PC71BM.
Effect of Tuning the Molar Mass Ratio
Tuning the molar mass ratio and annealing temperature of the blend aims to create a favourable nanoscale morphology in the heterojunction. Additionally, adjusting the cathode buffer layer aims to block excess holes from reaching the cathode, hence reducing charge-carrier recombination at the interface.
As the amount of PC71BM increased, the UV-Vis absorption of the blend was quenched, seen in the table below, which was attributed to the interaction between the donor and acceptor materials. A ratio of 1:0.8 P3HT:PC71BM was found to have the highest power conversion efficiency (PCE).
Emma's comments: The 1:0.8 P3HT:PC71BM ratio appears to be the best balance between high absorption and sufficient acceptor for efficient exciton dissociation.
Effect of Active Layer Annealing Temperature
From 100 to 150 °C, it was found that as annealing temperature was increased, UV-Vis absorption increased and was red-shifted. Above 150 °C, absorption decreased and blue-shifted, seen in the table below.
The initial increase in absorption was attributed to an increase in P3HT crystallinity, as a result of PC71BM diffusion caused by the higher temperature. This led to greater inter-chain interactions between P3HT chains, increasing the π-π* absorption and reducing the π-π* band gap causing the redshift.
The decrease in absorption after 175 °C was attributed to phase separation between donor & acceptor, also reducing the inter-chain interactions and hence causing a blueshift.
Emma's comments: The optimum annealing temperature depends on the blend's composition; the glass transition temperature is typically greater for compositions with a higher PCBM content. Heating a film blend too far above the glass transition temperature can reduce its performance.
Effect of Cathode Buffer Layer
In combination with the Al cathode, the cathode buffer layer was varied between LiF, Ca, tris (8-hydoxyqinolinato) aluminium (Alq3), Bathophenanthroline (BPhen) and Bathocuproine (BCP). These have varying band-gaps, which dictate their effectiveness. BCP was found to be the most effective layer.
Emma's comments: BCP has a higher band-gap than the other materials. Therefore it is more effective at hole blocking and reducing recombination. This illustrates the importance of matching band gaps throughout devices.
Final Thoughts
The work by Singh et al. clearly demonstrates the significant impact of processing conditions on bulk heterojunction morphology and determines the optimum conditions for P3HT:PC71BM. These findings are important to keep in mind during OPV manufacture in research, as conditions used for one stack may not be the most optimal for another. Several reviews are available to gain a deeper understanding of the impact of the donor and acceptor choice, and processing conditions, on the morphology and performance of bulk heterojunctions.3, 4, 5
Materials Mentioned in This Paper
If you are interested in conducting a similar experiment, some of these materials are available from Ossila.Learn More
 Organic Solar Cells: An Introduction to Organic Photovoltaics
Organic Solar Cells: An Introduction to Organic Photovoltaics
Organic solar cells, also known as photovoltaics (OPVs), have become widely recognized for their many promising qualities. This page introduces the topic of OPVs, how they work and their development.
Read more... Solar Cells: A Guide to Theory and Measurement
Solar Cells: A Guide to Theory and Measurement
A solar cell is a device that converts light into electricity via the ‘photovoltaic effect’. They are also commonly called ‘photovoltaic cells’ after this phenomenon, and also to differentiate them from solar thermal devices. The photovoltaic effect is a process that occurs in some semiconducting materials, such as silicon.
Read more...References
The paper discussed in this blogpost was published online in September 2017, and can be found here: http://www.sciencedirect.com/science/article/pii/S1566119917304743
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666-12731.
- A. Singh, A. Dey and P. K. Iyer, Organ. Electron., 2017, 51, 428-434.
- C. J. Brabec, M. Heeney, I. McCulloch and J. Nelson, Chem. Soc. Rev., 2011, 40, 1185-1199.
- C. J. Brabec, S. Gowrisanker, J. J. Halls, D. Laird, S. Jia and S. P. Williams, Adv. Mater., 2010, 22, 3839-3856.
- H. Hoppe and N. S. Sariciftci, J. Mater. Chem., 2006, 16, 45-61.