ITIC Molecule & Derivatives as OPV Acceptors
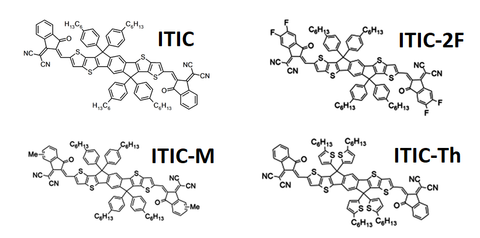
One of the most successful developments in non-fullerene acceptor (NFA) research was the creation of the fused-ring compound known as ‘ITIC’ (full form - 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakris(4-hexylphenyl)-dithieno[2,3-d:2’,3’-d’]-s-indaceno[1,2-b:5,6-b’]dithiophene) in 2015. In the initial paper proposing ITIC, a power conversion efficiency (PCE) of 6.80% was achieved in combination with a PTB7-TH donor.5 Efficiencies above 12% have been measured with various ITIC derivatives, such as ITIC-M,6 ITIC-2F7 and ITIC-Th.8
Why is ITIC a Good Acceptor?
ITIC performs well because of the extended conjugation in its core of fused aromatic rings which allows for rapid charge transport between molecules.9 Non-fullerene acceptors like ITIC - with a fused aromatic system - are known as fused-ring electron acceptors (FREAs). This also includes earlier NFAs, such as those based on rylene diimides.10
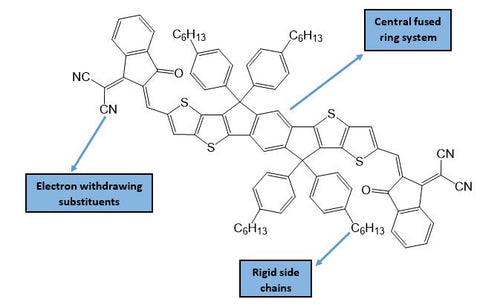
Typically, fused-ring systems will be modified with electron-withdrawing substituents (such as halogens) because these moieties lower the molecular energy levels of the acceptor, and allow energy-level matching with the electron donor.11 Rigid side-chains are also favourable, as these will prevent significant aggregation of the molecules, thus giving better bulk heterojunction (BHJ) morphology. Reduced aggregation is also a benefit of 3-dimensional acceptors like PCBM.2 This is in contrast to many planar acceptors that can form extended crystalline domains, which are unfavourable for BHJ morphology.
How Can We Make ITIC Better?
In the article, the authors modified ITIC with three different alkylthio-based side-chains, which can be seen in the figure below. This resulted in a yield of three ITIC analogues: ITIC-SC6, ITIC-SC8 and ITIC-SC2C6.
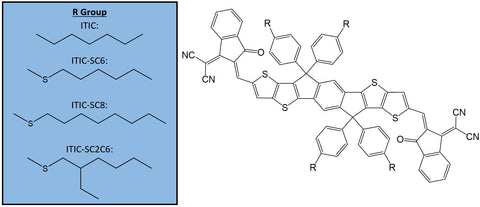
Emma's comments: Most common derivatives of ITIC (such as ITIC-Th) modify the core structure itself, but side-chain modification can also yield interesting results. The chemical nature of the side-chain, its position, bulkiness and length can all impact features such as solubility, intermolecular interactions, electronic properties and morphology.
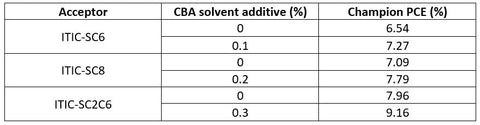
The impact on solar cell performance from each side-chain was assessed, and the results of this can be seen in the table below. The difference in results is proposed to be due to different solubility levels of the side-chains in the dichlorobenzene solvent. ITIC-SC2C6 showed the highest solubility in the solvent, and thus gave the most favourable BHJ morphology. Transmission electron microscope (TEM) images showed that with this acceptor, the donor was more likely to aggregate into nanofibril structures - giving an extended interpenetrating network, ideal for a BHJ active layer.
Final Thoughts
The main conclusion that can be drawn is that small changes in solubility of a molecule (due to alterations of side-chains) can have a significant impact on morphology. In order to gain optimum morphology for a BHJ cell, several side-chain combinations may need to be trialled, and the ideal acceptor could significantly increase the PCE of a system. Further investigation of the impact of side-chains on fused ring systems can be found in several other works.12-14
Materials Mentioned in This Paper
If you are interested in conducting a similar experiment, some of these materials are available from Ossila.
Learn More
 Organic Solar Cells: An Introduction to Organic Photovoltaics
Organic Solar Cells: An Introduction to Organic Photovoltaics
Organic solar cells, also known as photovoltaics (OPVs), have become widely recognized for their many promising qualities. This page introduces the topic of OPVs, how they work and their development.
Read more... Solar Cells: A Guide to Theory and Measurement
Solar Cells: A Guide to Theory and Measurement
A solar cell is a device that converts light into electricity via the ‘photovoltaic effect’. They are also commonly called ‘photovoltaic cells’ after this phenomenon, and also to differentiate them from solar thermal devices. The photovoltaic effect is a process that occurs in some semiconducting materials, such as silicon.
Read more...References
-
Effect of Non-fullerene Acceptors' Side Chains on the Morphology and Photovoltaic Performance of Organic Solar Cells, C. Zhang et al., ACS Appl. Mater. Interfaces, 9, 33906-33912 (2017); DOI: 10.1021/acsami.7b09915
-
Efficient Organic Solar Cells with Non-Fullerene Acceptors, S. Li et al., Small, 13, 2017; DOI: 10.1002/adma.201404317
-
Non-fullerene Electron Acceptors for Use in Organic Solar Cells, C. B. Nielsen et al., Acc. Chem. Res., 48, 2803-2812 (2015); DOI: 10.1021/acs.accounts.5b00199



