An Introduction to the Solid Electrolyte Interphase (SEI)

A solid electrolyte interphase (SEI) forms on the negative electrode in lithium-ion batteries (LIBs) due to the decomposition of electrolyte. The decomposition by-products build up on the surface of the anode and form an independent phase of material, different to the electrode and electrolyte. The SEI acts as a passivation layer which protects the electrolyte from further decomposition due to the high reactivity of lithium (Li0).
The solid electrolyte interphase (SEI) has a specific thickness, chemical composition, and morphology, depending on the battery components. It enables electrodes to operate beyond the electrolyte’s thermodynamic stability limit, preventing further degradation while allowing lithium ions to pass through to the anode. This allows lithium-ion batteries to charge and discharge reversibly over multiple cycles, contributing to their long lifespan and high cycle stability.
Solid Electrolyte Interphase Formation
There are two stages to the formation of the solid electrolyte interphase (SEI):
- When the anode becomes polarized, the electrolyte undergoes a reductive decomposition which generates new products
- The decomposition products precipitate and form the SEI across the whole anode surface.
The solid electrolyte interphase layer forms in lithium-ion batteries when the redox potential of the electrodes fall outside of the HOMO and LUMO energy levels of the solvent-electrolyte (eg. ethylene carbonate):
- Electron energies higher than the LUMO, the solvent-electrolyte is reduced
- Electron energy levels lower than the HOMO, the solvent-electrolyte is oxidized
Battery electrolyte is often more than just one solvent and contains salts (eg. LiPF6) and additives which also interact with the electrode surface and impact the redox potential of surrounding components. Therefore, the HOMO and LUMO energy levels alone do not provide enough information to predict the redox potential of the electrolyte.
The electrochemical stability of electrolytes is described as the potential of electrolyte reduction at negative potentials and the potential of solvent oxidation at positive potentials.
If the decomposition reaction potentials for the SEI formation are more positive than the anode lithium-ion intercalation potential, the solid electrolyte interphase formation is completed under fast kinetics before the start of intercalation of lithium ions. Most high-purity electrolyte solvents have a decomposition potential of 4.6-4.9 V vs Li/Li+, which is close to the preferred potential of 5 V for LIBs.
The electrochemical reaction rates for the formation of the SEI vary depending on the inherent properties of the electrolyte components and impurities (eg. water). These properties include:
Solid Electrolyte Interphase Composition and Structure
The solid electrolyte interphase is ~10-50 nm of products of electrolyte decomposition. The components of the SEI are heterogenous and form a disordered structure. Upon formation of the solid electrolyte interphase, kinetic stability is established.
It is difficult to determine the true composition of an solid electrolyte interphase as the structures are influenced by analyzing conditions. Exposure to air and moisture will affect the surfaces being analyzed with techniques such as scanning electron microscopy. XPS is used to determine the chemical composition of SEIs. The analysis must be carried out very carefully as results can vary with sample preparation method and the surface chemistry can change over time even under ultrahigh vacuum.
The chemical composition of the solid electrolyte interphase should be comprised of stable, insoluble, and compact compounds. The decomposed electrolyte products that form the SEI should not dissolve in the electrolyte to ensure high-capacity retention and SEI stability.
The surface of the electrode affects the composition, structure and thickness of the SEI. Taking the graphite anode as the example, the different planes of graphite layers see different SEI requirements. As lithium ions cannot intercalate into graphene layers across the basal plane ionic conductivity here is not required. Instead, electronic insulation and electrolyte blocking character is required without the SEI being too bulky. This is mostly comprised of organic species that arise from the decomposition of the carbonate solvent (eg. ethers).
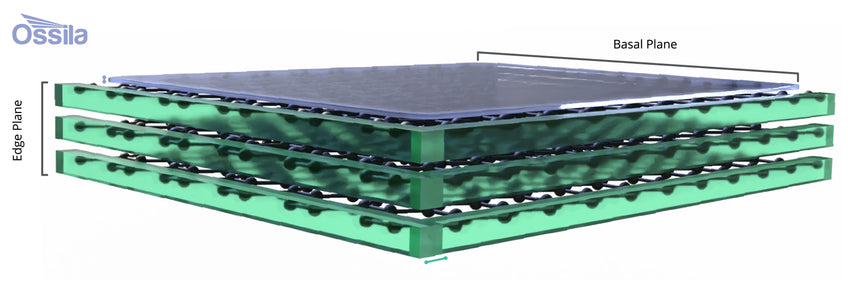
The edge planes contain dangling bonds, defects, functional groups and sp3 reactive sites. A denser and more homogeneous SEI layer is more likely to form. This site of SEI holds responsibility for having ionic conductivity to facilitate lithium-ion intercalation and electronically blocking. The SEI layer here is mostly comprised of inorganic species of carbonates and semicarbonates.
Solid Electrolyte Interphase Reactions
The reactions that take place during the formation of the solid electrolyte interphase are dependent on the components within the battery. Lithium ions are strongly solvated by solvent molecules. Solvents preferentially coordinate to lithium and outcompete anions. The concentration polarization causes solvated lithium ions to diffuse toward the anode surface. The various suggested reaction pathways at a graphite anode include:
- Intercalation of desolvated lithium ions into the graphene layers
- Heterogeneous transfer of electrons from the graphite electrode to the solvent molecules
- Co-intercalation of the solvent molecules and the solvated lithium ions into the graphene layers
- Heterogeneous transfer of electrons from the graphite electrode to the salt anions.
Each component is involved in a different type of reaction:
- Solvents – reduction of carbonate solvents follows either a one-electron or two-electron reduction process to produce SEI components.
- Solvated Lithium-ions – intercalate into the graphene layers and undergo reduction reactions to produce SEI components
- Salt – can interact with solvent molecules and solvent decomposition products to form SEI components. Can also react with impurities to form harmful species such as HF and HPO2F2.
- Impurities – can react with lithium ions to form SEI components.
SEI species formed using standard carbonate-based electrolytes:
| Soluble Species | Insoluble Species | Impurities |
|---|---|---|
|
Ethers Fluorophosphates Oligioethylene oxides |
LiF Li2CO3 Li2O Lithium carboxylates Lithium alkoxides Lithium Fluorophosphates |
CO2 Ethylene HF PF5 HPO2F2 |
Solid Electrolyte Interphase Properties
For rechargeable lithium-ion batteries, the SEI must exhibit ionic conductivity while acting as an electronic insulator. This ensures efficient ion transport while preventing unwanted electron flow, which could lead to further electrolyte decomposition and battery degradation.
Functions of a stable SEI
A complete and stable SEI provides several crucial benefits:
- Restricts Electron Tunnelling – If the SEI is thicker than 1 nm, it effectively prevents electron tunnelling. This helps protect the electrolyte from unwanted reduction reactions, which can decrease battery efficiency and lifespan.
- Prevents Electrolyte Reduction – By acting as a protective barrier, the SEI stops further decomposition of the electrolyte on the anode surface. This stabilizes battery performance over multiple charge-discharge cycles.
Ideal SEI Properties
For long-term battery performance, an ideal SEI should possess the following characteristics:
- Chemically Stable Against Lithium – The SEI should not react with lithium metal or lithium ions, ensuring long-term stability.
- High Lithium-Ion Conductivity but Negligible Electronic Conductivity – It must facilitate lithium-ion transport while blocking electron movement. This prevents continuous electrolyte degradation and unwanted side reactions.
- Strong and Flexible – The SEI must withstand the mechanical stress caused by anode volume expansion during charging and contraction during discharging. A flexible yet durable SEI prevents cracking and maintains structural integrity over time.
By maintaining these properties, the SEI enables lithium-ion batteries to achieve high efficiency, long cycle life, and stable performance across multiple charge-discharge cycles.
Challenges of Solid Electrolyte Interphase Layers
Investigating battery solid electrolyte interphase layers is a particularly challenging area of research. Understanding the structure-property relationship between the electrode and electrolyte materials is vital in optimizing lithium-ion battery technology. However, there is a severe lack in experimental techniques which work on the required nanoscale and resolution required to characterize the SEI structure and properties. To avoid exposure to conditions which alter the integrity of the SEI layer, non-destructive and in situ techniques must be realised.
Anode Active Materials
Learn More
The NCA battery gets its name from the cathode active material, lithium nickel cobalt aluminum oxide (LiNixCoyAlzO2, where x+y+z=1) which gets shortened to nickel cobalt aluminum (NCA). NCA is the cathode active material with a specific ratio of metals.
Learn more... Graphene Battery vs Lithium-Ion Battery
Graphene Battery vs Lithium-Ion Battery
Lithium-ion (Li-ion) batteries, developed in 1976, have become the most commonly used type of battery. They are used to power devices from phones and laptops to electric vehicles and solar energy storage systems.
Read more...References
- Solid electrolyte interphases in lithium metal batteries, Jagger, B. et al., Joule (2023)
Further Reading
- Adennusi, H. et al. (2023). Lithium Batteries and the Solid Electrolyte Interphase (SEI)—Progress and Outlook, Advanced Energy Materials, doi:10.1002/aenm.202203307
- Xu, K. (2023). Interfaces and interphases in batteries, Journal of Power Sources, doi:10.1016/j.jpowsour.2023.232652
Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer