The Allotropes of Phosphorus
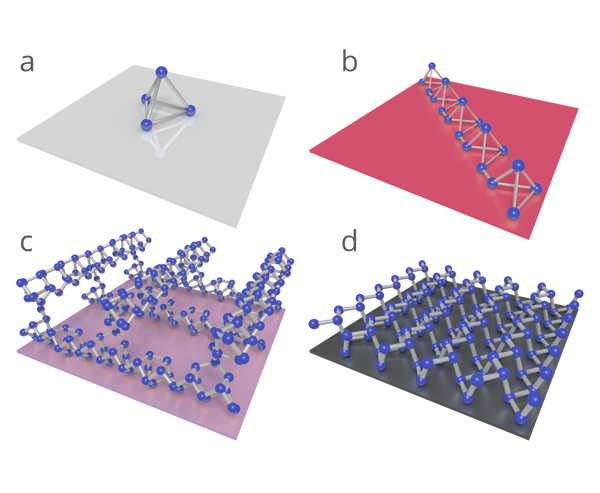
Depending on environmental conditions and how it is processed, elemental phosphorus can take several forms (allotropes). These allotropes are generally referred to by their physical appearance; white (or yellow) phosphorus, red phosphorus, violet phosphorus, and black phosphorus.
White Phosphorus
White phosphorus will auto-ignite and burn fiercely in air when heated to 30 °C, which limits its use in pure form to explosives. It is a wide bandgap insulator, and this — combined with its tendency to explode — is the reason why it has not garnered much interest from the optoelectronic industry.
Red Phosphorus
Red phosphorus is a more stable allotrope (note that several different forms of red phosphorus exist, with varying degrees of crystallinity) that displays some semiconducting properties (Wang et al., 2012). Despite having a bandgap in the near-infrared (NIR), red phosphorus is also yet to find an application in semiconductor devices. It is, however, used on the striking surface of ‘safety’ matchboxes. Previously, ‘non-safety’ matches used white phosphorus as the ignition source.
Violet Phosphorus
Violet phosphorus (or ‘Hittorf phosphorus’, after the person who first discovered it) is a stable crystalline allotrope that takes the structural form of nanotubes with a pentagonal cross-section. It is sometimes classed as a subset of red phosphorus. Despite being isolated in 1865, relatively little is known about it. Some recent theoretical work suggests that monolayers of violet phosphorus could exhibit a larger direct bandgap than black phosphorus with good charge carrier mobility (Schusteritsch et al., 2016). This could be a material to watch out for in the near future.
Black Phosphorus Compared to Phosphorus Allotropes
Black phosphorus differs considerably from other allotropes of phosphorus, which do not share its stability, properties, or structure.
The properties of the four common allotropes of phosphorus are summarised in the following table.
| White (or Yellow) | Red | Violet (or Hittorf/Monoclinic) | Black (or Phosphorene) | |
|---|---|---|---|---|
| Appearance at Room Temperature | White solid | Red solid | Purple solid. Large pieces appear glossy | Black solid |
| Structure | Tetrahedron of 4 P atoms | Amorphous structure of covalently-bonded P atoms | Crystalline pentagonal nanotubes of covalently bonded P atoms | Van der Waals bonded layers of P atoms covalently bonded in a puckered honeycomb structure |
| Synthesis | Heating of phosphate rock with carbon and silica to ~1200 °C | Heating of white phosphorus to ~300 °C in an air-free environment | Heating and cooling of white phosphorus dissolved in lead, or heating of red phosphorus in a sealed tube | Heating of white phosphorus to ~200 °C at 1.2 GPa, or from red phosphorus at 8.5 GPa |
| Properties | Will auto-ignite when exposed to air at ~30 °C. Wide band gap insulator. Chemiluminescent (but not phosphorescent) | Will auto-ignite when exposed to air at ~300 °C. Semiconductor | More stable and more dense than red phosphorus. Monolayers may exhibit a direct band gap larger than black phosphorus | Most stable allotrope of phosphorus. Semiconductor. Thickness-dependent bandgap. High charge carrier mobility |
| Uses | Flares, explosives | Matches, flame retardant (when mixed with flammable plastics at low concentration) | Little is currently known about the optoelectronic properties of violet phosphorus | Potential applications in optical devices (eg. photodetectors), electronics (eg. FETs), energy storage, and many more |
The structure of each allotrope of phosphorus is shown below.
Black Phosphorus

Learn More
 Black Phosphorus Uses and Applications
Black Phosphorus Uses and Applications
Due to the unique properties, exfoliated monolayer and few-layer black phosphorus have potential for a wide range of applications in electronics and optoelectronics.
Read more...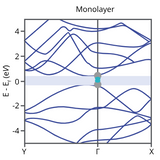 Black Phosphorus Properties
Black Phosphorus Properties
The optical, electronic, and mechanical properties of phosphorene differ from that of the bulk state due to combinations of factors.
Read more...References
- Red Phosphorus: An Elemental Photocatalyst for Hydrogen Formation from Water, F. Wang et al., Appl. Catal., 111, 409-414 (2012)
- Single-Layered Hittorf’s Phosphorus: A Wide-Bandgap High Mobility 2D Material, Schusteritsch et al., Nano Lett., 16 (5), 2975-2980 (2016)