What are Self-Assembled Monolayers (SAMs)?
A self-assembled monolayer (SAM) is a well-organised, one molecule thick layer that forms on a solid surface. SAM molecules, like MeO-2PACz, adsorb on a substrate via chemical or physical bonds. They spontaneously organize themselves into ordered structures on the substrate. SAM materials have gained interest due to their potential applications in various fields such as solar cell research, nanotechnology, materials science, chemistry, and biology.
Structure of Self-Assembled Monolayer Molecules
The molecules in typical self-assembled monolayers consist of three main components:
- Anchor / Head: binds to a solid substrate
- Linker / Spacer: varies in functionality to build up a barrier between the head and the terminal group
- Terminal group: determines the exposed surface/interface properties
The combination of these three components influences the SAM molecule’s properties. Altering the components allows for the fine tuning of surface energy, wettability, charge mobility, and reactivity to achieve the desired surface properties of the substrate. Among the most studied are alkanethiol self-assembled monolayers on gold substrates.
Head / Anchor
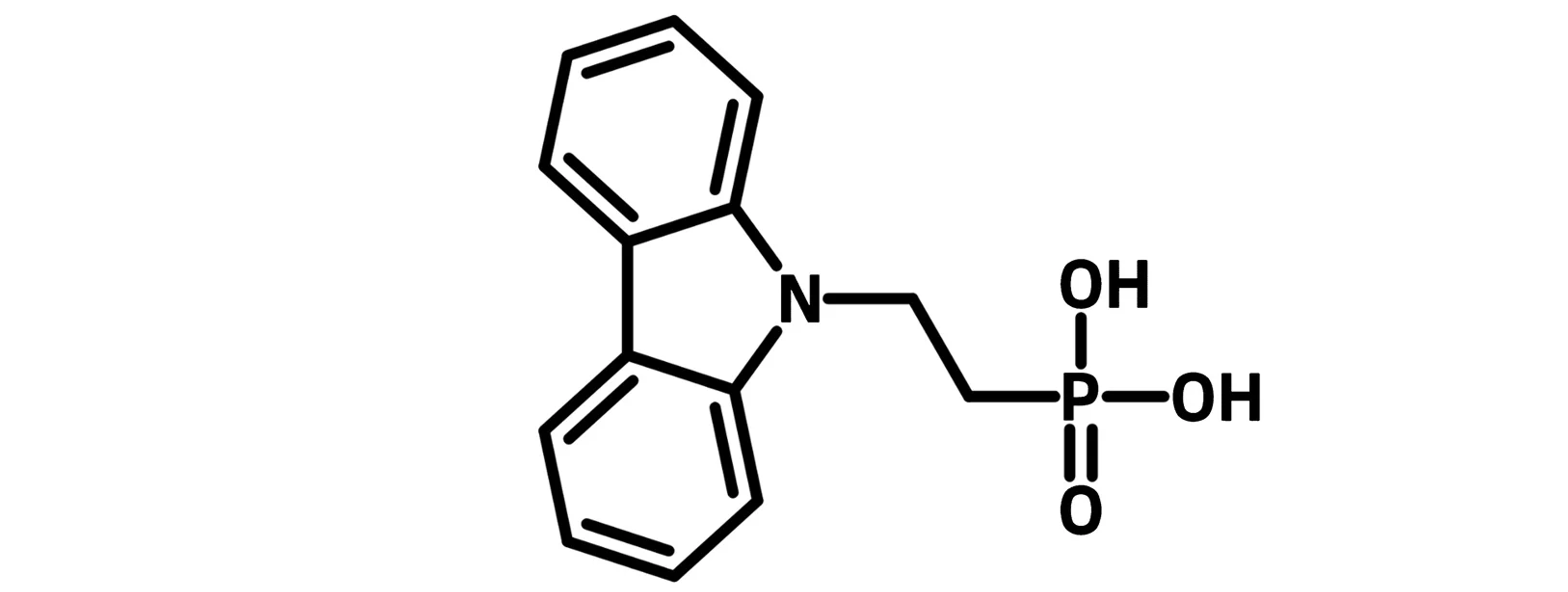
The head group serves as the anchor that connects the self-assembled monolayer material to the substrate. They modify the coverage ratio, the contact resistance, and the work function of the substrate. Common head groups are typically hydrophilic:
| SAM Head Group | Solid Surface |
|---|---|
|
Phosphonic acid (-PO3H2), Hydroxyl (-OH), Sulphuric acid (-SO3H) |
Metal Oxides |
|
Thiols (-SH) |
Gold |
|
Silanes (-SiH3) |
Silicon |
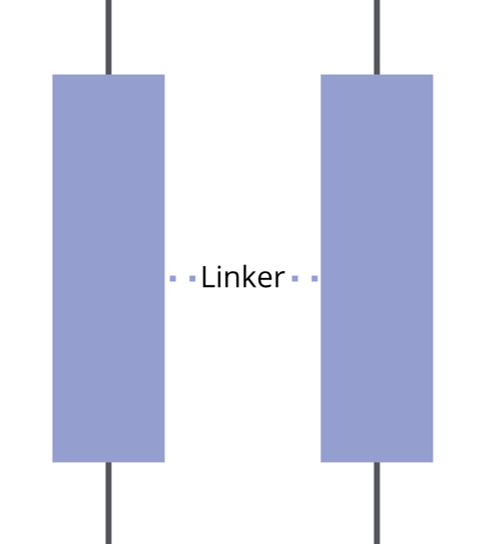
Spacer / Linker
The SAM spacer is normally a hydrophobic alkyl chain of different carbon length, with or without phenyl rings. It provides structural support and determines the packing density and orientation of the molecules.
Terminal Group
The SAM tail is either an electron rich group such as carbazole, dibenzo[c,g]carbazole, phenothiazine, 9,9-dimethyl-9,10-dihydroacridine, triphenyl amines, or electron deficient group such as naphthalene diimide. It often bears functionalities such as hydroxyl, amine, carboxylic acid groups. The functional terminal group modifies the surface and interface properties.
Example Self-Assembled Monolayer Molecules
Self-Assembled Monolayer Formation
The process of self-assembly is driven by thermodynamic forces. This include van der Waals interactions among the alkyl chains and the specific binding interactions between the head groups and the substrate.
Self-assembled monolayers (SAMs) spontaneously form from solution or vapour phase. This is done by combining the advantages of ultra-high vacuum (UHV) and the Langmuir–Blodgett method:
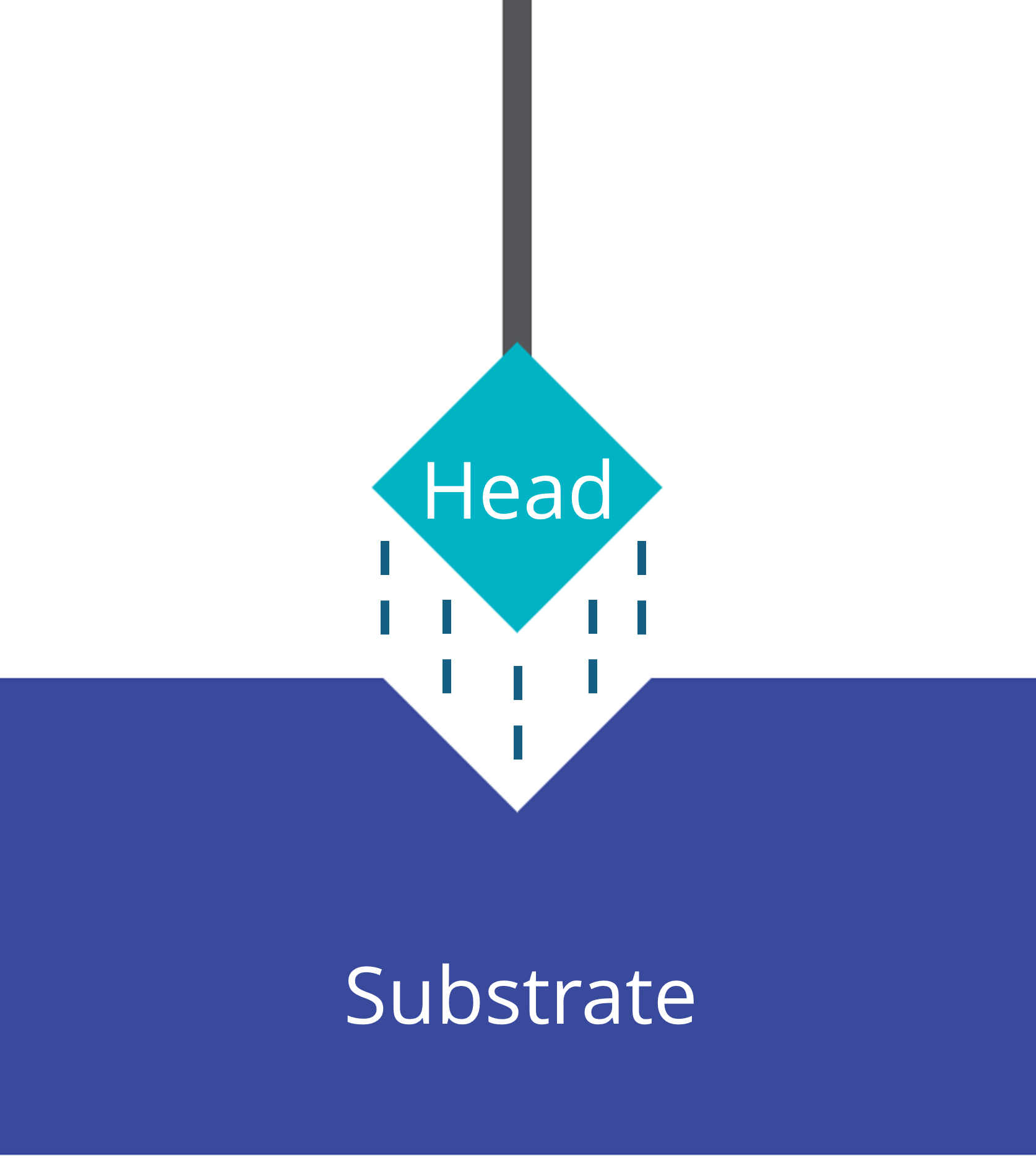
UHV-formed films show strong interaction between the substrate and the head groups by chemisorption. For example, strong gold-sulfur bonds form when thiol heads are exposed to a gold substrate.
In Langmuir–Blodgett films, chain–chain physisorption interactions (involving van der Waals and hydrophobic forces) ensure an efficient packing of the monolayer. Stability is increased with increasing chain length.
Self-Assembled Monolayer Uses
Self-assembled monolayers represent a versatile and powerful tool in surface science and nanotechnology. Their ability to form ordered structures with tailored properties makes them suitable for a wide range of applications, from electronics and catalysis to biotechnology:
| Application | Description |
|---|---|
| Protective Layer | SAMs can protect metal surfaces by creating a barrier that inhibits the interaction between the metal and corrosive agents. |
| Catalysis |
SAMs support catalytic materials or to create surfaces with specific catalytic properties. They enhance the selectivity and efficiency of catalytic processes by providing a well-defined environment for reactions. |
| Biomedical | SAMs are used in the design of medical implants, and tissue engineering scaffolds by creating surfaces to promote or inhibit cell adhesion. |
| Biosensing |
SAMs facilitate the immobilization of biomolecules such as peptides and antibodies for biosensing applications. |
| Photovoltaics | SAMs are popular candidates for hole/electron selective contacts in photovoltaic solar cells. Particularly in perovskite solar cells, SAMs boost device efficiency and improve stability. |
Current Limitations of Self-Assembled Monolayers
Despite their promising advantages, self-assembled monolayers can degrade over time. Especially in harsh environments. Exploring different head groups and tail functionalities to expand the range of properties and stability are key to the next stages of research. Developing effective self-assembled monolayer materials will be beneficial to applications ranging from organic electronics to medical biology.
The presence of defects such as vacancies or disordered regions can affect the performance of SAMs. Advanced fabrication techniques are needed to minimize these defects and achieve uniformity. For commercial applications, the scalable production of high-quality SAMs is crucial. This requires efficient and cost-effective methods for large-scale fabrication.
Self-Assembled Monolayers (SAMs)
Learn More
 Deposition of Self-Assembled Monolayers
Deposition of Self-Assembled Monolayers
Self-assembled monolayers (SAMs) are well-organized one molecule thick layers that spontaneously form on a surface. They have a high potential for use in a wide range of applications due to their well-defined structure and properties.
Read more... Applications of Self-Assembled Monolayers
Applications of Self-Assembled Monolayers
Self-assembled monolayers (SAMs) are highly organized single layers of molecules that form on a surface. This is driven by the chemisorption of specific functional groups on the SAM molecules that have a strong affinity for a particular surface.
Read more...References
- F. Schreiber (2004). Self-assembled monolayers: from ‘simple’ model systems to biofunctionalized interfaces, J. Phys.: Condens. Matter,DOI: 10.1088/0953-8984/16/28/R01
- C. Vericat et al. (2005); Self-assembled monolayers of alkanethiols on Au(111): surface structures, defects and dynamics, Phys. Chem. Chem. Phys., 7, DOI: 10.1039/B505903H
- D. Utomo et al. (2024); Nonfullerene Self-Assembled Monolayers As Electron-Selective Contacts for n-i-p Perovskite Solar Cells, ACS Energy Lett., 9 (4), DOI: 10.1021/acsenergylett.4c00306




