What is an Electrochemical Cell?

An electrochemical cell is defined as a device that generates electrical energy from chemical reactions or uses electrical energy to drive chemical reactions. The simplest possible electrochemical cell consists of two connected electrodes in an electrolyte solution. The two electrodes are:
- Anode: where oxidation reactions (loss of electrons) occur
- Cathode: where reduction reactions (gain of electrons) occur
Current flows between the electrodes, allowing various experiments to determine the electrochemical properties of the components within the cell. Examples include measuring the oxidation and reduction potentials of battery materials.
Types of Electrochemical Cell
There are two main types of electrochemical cell:
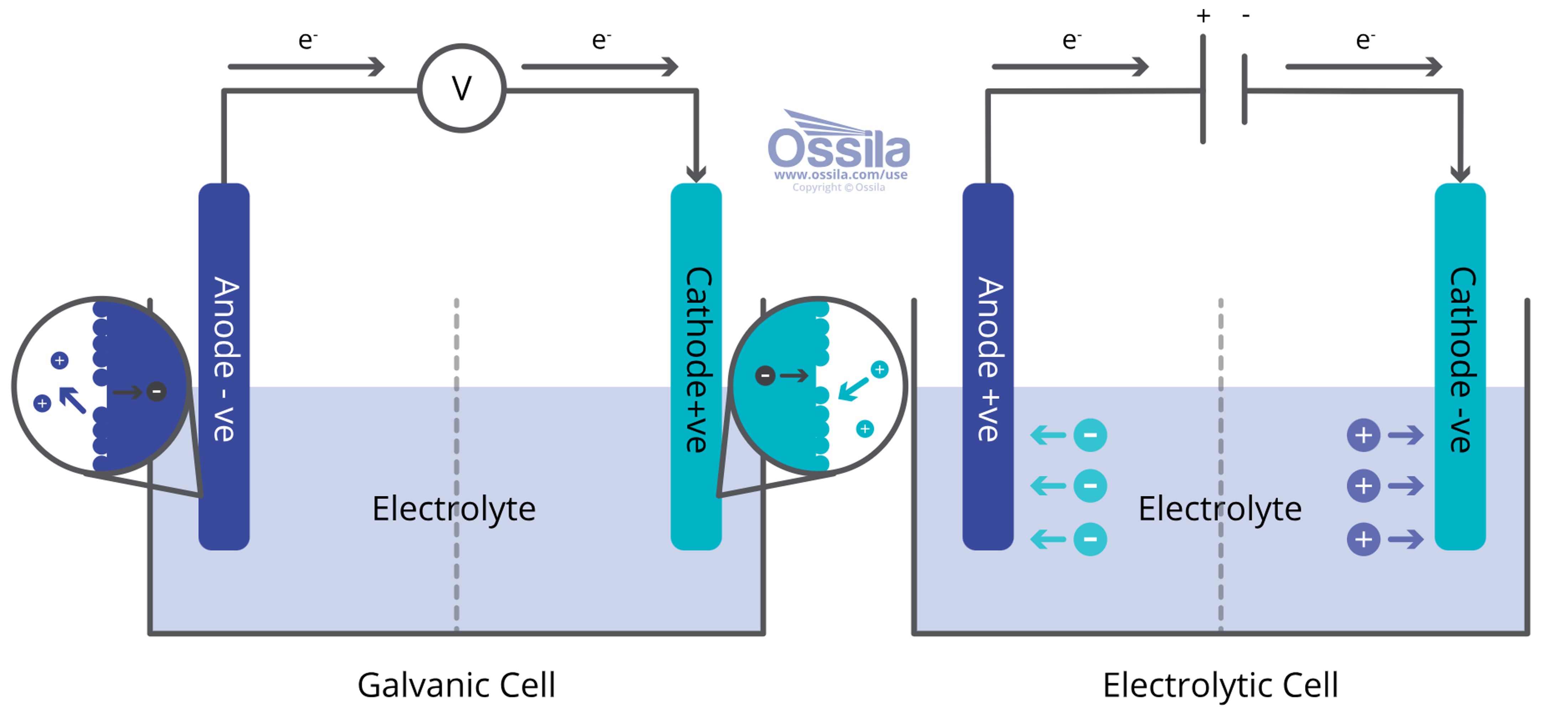
Galvanic/Voltaic Cell
- Generates electricity from spontaneous chemical reaction.
- Anode is negative and releases electrons which flow through an external circuit to cathode, generating an electric current.
- Common examples are standard AA batteries and fuel cells.
Electrolytic Cell
- Drives non-spontaneous chemical reaction.
- Externally applied voltage forces a chemical reaction.
- The anode is positive and the cathode is negative.
- Applications include electroplating where the electric current reduces dissolved metal cations to form a thin coating and electrolysis of water.
Three Electrode Electrochemical Cell
An electrochemical cell can have a variety of different electrode setups. A common setup for electrochemical experiments involves three electrodes. This setup is often used in potentiostatic or galvanostatic measurements. However, an electrochemical cell with two electrodes is more typical for practical applications like batteries and fuel cells. For a three electrode system, a different naming system is used:
Working electrode: where the reaction of interest takes place (either oxidation or reduction). The potential is controlled and measured against a reference electrode. It is commonly made from platinum, glassy carbon or gold, amongst other materials.
Counter electrode: completes the circuit by allowing the current to flow. Does not take part in the reaction of interest. Used to balance the current at the working electrode.
Reference electrode: provides a stable reference potential for the working electrode.
A luggin capillary is a small tube filled with electrolyte in which the reference electrode is placed. It is positioned close to the working electrode to minimize uncompensated resistance. This improves the accuracy of potential measurements by minimizing potential errors.
Why Do we Use Electrolyte in Electrochemical Cells?
Inert ions are added in excess to the electrolyte solution to increase its ionic strength, ensuring it follows the Nernst equation. The Nernst equation is fundamental in electrochemistry and relates the electrode potential during analysis to the electrode potential under standard conditions, temperature, and activities (or concentrations) of the chemical species involved.
The excess electrolyte reduces the thickness of the diffuse double layer, causing the applied potential to drop to a negligible level within nanometers of the working electrode. This results in a well-defined current response at the electrode surface, enhancing the accuracy and reliability of the measurements and ensuring the system behaves according to the theoretical predictions of the Nernst equation.
Example Electrochemical Cells
What are Electrochemical Cells Used for?
Analytical electrochemical cells are used for a wide range of applications that require the detection, analysis and quantification of chemicals. The electrochemical properties of various substances can be determined. Here are some common uses:
- Electrochemical Sensors: pH meters, glucose sensors
- Cyclic Voltammetry: redox potentials and reaction kinetics
- Electroanalytical Methods: potentiometry, amperometry, coulometry
- Corrosion Studies
- Battery and Fuel Cell Research
- Electroplating and Electrolysis
- Chemical and Biological Analysis
- Environmental Monitoring
These applications are possible because components of the electrochemical cell can be varied depending on the desired data output. Here are some of those variables:
| Electrical | Mass Transfer | External | Electrochemical Analyzer | Electrode | Solution |
|---|---|---|---|---|---|
|
Potential (E) Current (i) Quantity of Electricity (Q) |
Diffusion Surface concentrations Adsorption Flow Rate |
Temperature Pressure Time |
Potentiostat Galvanostat Impedence Analyzer |
Material Surface Area Geometry Surface Condition |
Bulk concentration of electroactive species Concentration of other species Solvent |
Electrochemical Cells

Electrochemical Cell Setup
The physical setup of an electrochemical cell is relatively simple. The working and counter electrodes sit in an electrochemical solution, and the reference electrode sits in a separate tube within the cell containing the reference solution. The reference electrode tube should be approximately two thirds full - a syringe and needle can be used to add the solution.
Degassing
In addition to having holes for each electrode, electrochemical glassware typically has gas intakes to allow for an inert gas (usually nitrogen or argon) to be bubbled through the solution to remove its oxygen. This process is known as degassing.
Degassing is important because molecular oxygen is electrochemically active, and if not removed will create unwanted redox processes. In addition, the products of this reaction (hydrogen peroxide) can also interact with the compound and further interfere with the results of the experiment.
Once you remove the oxygen, you can keep it out with a continuous stream of inert gas.
Risk of Contamination
When you prepare an electrochemical cell, it is important to minimise the risk of any contamination with water. This is because water can form reactive species when reduced or oxidised. You can do this by heating the components in a glassware oven prior to using them and carrying out the measurements in a mositure-free, inert glove box environment.
Electrochemical Solutions
Electrochemical solutions typically consists of three components.
- The compound of interest (10-3 – 10-5 M)
- An electrolyte (0.1 M)
- A solvent which dissolves both the compound of interest and the electrolyte
Solvent Choice
Your choice of solvent and electrolyte should be dictated by the solubility of your studied chemical (so that it can be dissolved at the concentration needed) and the desired potential range.
Reference tables of the potential range of various solvent and electrolyte pairs are widely available [1] but these ranges are highly dependent on the purity and dryness of both the electrolyte and solvent. For the best results, choose a high purity solvent and electrolyte and oven dry all components before use.
Be aware when manually purifying and drying your components by standard procedures [2] that Grubbs purification apparatuses can add undesirable electroactive impurities [3].
Solvent Reference Table
Potential ranges is shown below based on the values given by A.J. Bard and L.R. Faulkner [2]. Values are given relative to the Standard Calomel Electrode (SCE).
Aqueous Solvent Systems
Non-aqueous Solvent Systems
Insoluble Chemicals
It is possible to study the electrical response of materials, like polymers, which cannot be sufficiently dissolved in standard electrochemical solvents. To do this, we recommend that you coat the working electrode with the material by depositing it with a solvent.
Due to the complex nature with which charges diffuse through the solid and the various distortions which occur within the deposited compound, the normal equations and mathematical proofs do not strictly apply under these circumstances. By approximating the onset potential as the redox potential of that process, however, the technique still gives a good approximation of the energy levels for insoluble materials.
Internal Standards
You can use internal standards, usually ferrocene, to calculate the value of the oxidation and reduction potentials. Internal standards are compounds which oxidise or reduce in solution, ideally somewhat independently of the system (although ferrocene does vary between solutions).
This oxidation or reduction provides a voltammogram which you can use to reference the position of the oxidation or reduction of the compound of interest.
It is common practice to study these standards immediately after the chemical of interest, using the same solutions. Recent reviews, however, suggest that it is better to always have the internal standard present in order to prevent changes in the position of the voltammograms [6]. This is particularly true for quasi reference electrodes where large shifts have been observed.
Choice of Electrodes
Working and Counter Electrodes
The counter electrode and working electrode must be conductive so that charges can move to and from the solution, and they must not cause any chemical reaction in the solution. You can usually achieve inertness by making them out of unreactive material such as platinum.
A large counter electrode surface area makes sure that the measured current corresponds to the current flow between the working and counter electrode [5].
Choice of Reference Electrode
Reference electrodes are designed so that an equilibrium is setup with known potential between the metal wire and the surrounding solution. The reference electrode is setup in the cell so that it is in a circuit with the counter electrode and working electrode in opposing directions. In one direction, the working electrode goes from the solid state into the solution and the reference electrode goes from the solution to the solid state.
The consequence of this (along with Kirchhoff's voltage law and zero solution resistivity) is that the measured potential is zero when the working electrode potential is equal to the reference electrode potential.
The most common reference electrodes are the standard calomel electrode, the normal hydrogen electrode, the silver/silver chloride (Ag/AgCl) electrode in saturated potassium chloride and the Ag/Ag+ (0.01M, usually AgNO3) electrode in acetonitrile. Their standard reduction potentials are listed below.
It should be noted that the Ag/Ag+ electrode is usually setup with the same electrolyte solution that is used in the studied solution. This is to minimise the junction potentials (the potential between the reference solution and the studied solution). You should take this into consideration when choosing your electrolyte and your solvent as well as when estimating the volume of solution that you require for your experiment.
| Electrode | Standard reduction potential / eV |
|---|---|
| Normal Hydrogen Electrode | 0.000 (by definition) [1] |
| Standard Calomel Electrode | 0.242 [1] |
| Ag / Ag+ 0.01 M (usually AgNO3) in CH3CN | Variable dependent on setup [5] |
| Ag/AgCl, KCl(sat. in H2O) * | 0.197 [1] |
* Note: the AgCl coats the silver electrode
The reference electrode is setup so that the reference solution is separated from the studied solution via a frit.

This arrangement allows an electrical connection which permits a measurement of voltage. The slow movement of liquid through the frit (a porous glass membrane that allows liquid to flow through it at a slow rate) reduces the mixing of the reference solution and the studied solution to a minimum.
Even with the use of a frit, however, some mixing to be expected. For this reason, an Ag/Ag+ electrode is sometimes favoured over the Ag/AgCl, KCl(sat. in H2O). This is partly becuase the Ag/AgCl, KCl(sat. in H2O) will slowly leak water over time and water impurities in the studied solution lead to the narrowing of the potential window.
In addition, the AgCl in solution may also be reactive to certain studied chemicals. A double frit can be employed to prevent this, with an interior reference solution and an exterior studied solution separated from the bulk studied solution. This prevents the studied solution near the working electrode from being contaminated with water.
Note: Frits should always be stored in liquid between uses to prevent degradation. Never store a frit in air.
The Quasi-reference Electrode
An alternative reference electrode in electrochemical experiments is the quasi-reference electrode (also known as a pseudoreference electrode). This is a reference electrode (usually silver wire) which does not have a surrounding solution with ions to form the half equation.
Because the potential of this reference electrode is not defined by ions of known concentration, the use of an internal standard such as ferrocene is vital. In addition, because the point which is being referenced against can shift depending on the contents of the solution, it is important to ensure that the internal standard is present during the reduction / oxidation of the studied chemical.
There are some disadvantages to using a quasi-reference electrode. While they can reproduce the results of a standard reference electrode and are much easier to setup, they are also much more susceptible to potential drift [6].
A large standard deviation has also been reported when using a quasi-reference electrode. This can be reduced by separating the electrode from the rest of the solution using a frit (with the reference solution the same as the studied solution) [6][7].
Learn More
A redox reaction, also referred to as an oxidation-reduction reaction, involves the loss or gain of electrons. The loss of electrons is called oxidation and the gain of the electrons reduction.
Read more... What is a Photoelectrochemical Cell?
What is a Photoelectrochemical Cell?
A photoelectrochemical cell (PEC) is a device that converts solar energy (light) into chemical energy or electricity. Light activates a semiconductor or photosensitizer component within the cell.
Read more...References
- Sevćik, A. Collection of Czechoslovak Chemical Communications 1958, 13, 349
- A. L. Bard and L. Faulkner Electrochemical methods: Fundamentals and Applications, 2nd ed. John Wiley & Sons 2001
- W. L. G. Armarego and C. L. L. Chai Purification of Laboratory Chemicals, 7th ed. Butterworth-Heinemann 2012
- L. J, L. B, and P. G Advanced Practical Organic Chemistry, 3rd edition. Manipal: Routledge 2013
Contributing Authors
Written by
Application Scientist
Software Engineer
PhD Student Collaborator
Product Expert
Diagrams by
Graphic Designer