1,4-Dibromonaphthalene
CAS Number 83-53-4
Chemistry Building Blocks, Dibromo Monomers, Materials, Monomers, Non-Heterocyclic Building BlocksA well known intermediate used for the synthesis of functional materials
Used for drug discoveries, coordination chemistry and organic electronic devices.
Specifications | MSDS | Literature and Reviews
1,4-Dibromonaphthalene (1,4-DBN), CAS number 83-53-4, is a bis-brominated naphthalene at 1,4-positions. 1,4-dibromonaphthalene can be obtained by the treatment of 1-bromonaphthalene with bromine in dichloromethane at -30 °C in high yield.
1,4-Dibromonaphthalene is well known as a triplet excitation acceptor with useful phosphorescent properties. It is also an useful intermediate for the synthesis of other 1,4-dibromonaphthalene derivatives in drug discovery such as enzyme-inhibitory, antifungal, antibacterial, anticancer, anti-inflammatory, antiallergic, phenols, amines, coordination chemistry and organic electronic devices. Ambipolar deep-blue emitter, 4,4′-bis(4-(1-(4-(tert-butyl)phenyl)-1H-phenanthro[9,10-d]imidazol-2-yl)phenyl)-1,1′-binaphthalene (2NBTPI), derived from 1,4-dibromonaphthalene, demonstrates spatially separated HOMO and LUMO orbits, a high thermal stability and deep blue emission in OLED device.
Naphthalene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Brominated at 1,4 positions
for facile reactions
High purity
>98% Purity
General Information
| CAS Number | 83-53-4 |
| Chemical Formula | C10H6Br2 |
| Full Name | 1,4-Dibromonaphthalene |
| Molecular Weight | 285.97 g/mol |
| Synonyms | 1,4-DBN |
| Classification / Family | Naphthalenes, Semiconductor synthesis intermediates, Low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
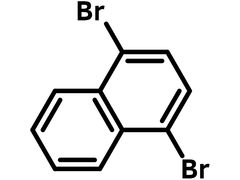
Product Details
| Purity | >98% (1H NMR) |
| Melting Point | Tm = 82 °C |
| Appearance | White to off-white powder/crystals |
MSDS Documentation
 1,4-Dibromonaphthalene MSDS Sheet
1,4-Dibromonaphthalene MSDS Sheet
Literature and Reviews
- Highly efficient desymmetrisation of a tricarbonylchromium 1,4-dibromonaphthalene complex by asymmetric Suzuki–Miyaura coupling, X. Urbaneja et al., Chem. Commun., 47, 3739-3741 (2011); DOI: 10.1039/C1CC10347D.
- An environment-friendly method for synthesis of 1,4-dibromo-naphthalene in aqueous solution of ionic liquids, X. Zhao et al., Catal. Commun., 9(13), 2179-2182 (2008); DOI:10.1016/j.catcom.2008.04.020.
- Intrachain and interchain triplet-triplet exciton annihilation in a quasi-one-dimensional crystal: 1,4-dibromonaphthalene (DBN), H. Bouchriha et al., Chem. Phys. Lett., 53 (2), 288-293 (1978); DOI: 10.1016/0009-2614(78)85398-6.

