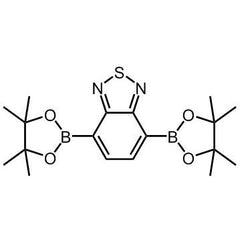
2,1,3-Benzothiadiazole-4,7-bis(boronic acid pinacol ester)
CAS Number 934365-16-9
Boronates, Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, MonomersHigh-purity monomer for the synthesis of small molecules, oligomers and polymers
Available online for fast, secure dispatch
Specifications | MSDS | Literature and Reviews
2,1,3-Benzothiadiazole-4,7-bis(boronic acid pinacol ester), CAS number 934365-16-9, is an intermediate for the synthesis of small molecules, oligomers and polymers via Suzuki Coupling reactions. Those small molecules, oligomers and polymers can be printed, spray-coated or spin-coated onto devices for Organic Light-Emitting Diodes (OLED/PLED), Organic Field-Effect Transistors and Organic Photovoltaic devices.
2,1,3-Benzothiadiazole unit is electron deficient so it is employed for the low-band gap semiconducting materials used for OLED and OPV devices, i.e. F8BT and PCPDTBT.
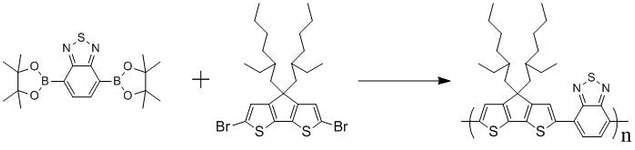
Capped with Bpin
A monomer for F8BT and PCPDTBT
Benzothiadiazole building block
For semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 934365-16-9 |
| Chemical Formula | C18H26B2N2O4S |
| Molecular Weight | 388.10 g/mol |
| Synonyms | 2,1,3-Benzothiadiazole-4,7-diboronic acid bis(pinacol) ester Benzo[c]-1,2,3-thiadiazol-4,7-diyl-4,7-diboronic acid dipinacol ester 4,7-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,1,3-benzothiadiazole 4,7-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[c][1,2,5]thiadiazole |
| Classification / Family | Benzothiadiazole, Heterocyclic five-membered ring, Organic semiconducting materials, Semiconductor Synthesis, Low band gap polymers, OFETs, OLED, Organic Photovoltaics, Polymer solar cells |
Chemical Structure
Product Details
| Purity | >98% |
| Melting Point | 213 °C - 215 °C |
| Appearance | White/Off-white powder |
NMR Characterisation
MSDS Documentation
 2,1,3-Benzothiadiazole-4,7-bis(boronic acid pinacol ester) MSDS sheet
2,1,3-Benzothiadiazole-4,7-bis(boronic acid pinacol ester) MSDS sheet
Literature and Reviews
- Combination of Molecular, Morphological, and Interfacial Engineering to Achieve Highly Efficient and Stable Plastic Solar Cells, C-Y. Chang et al, Adv. Mater., 24, 549-553 (2014)
- Development of Polymer Acceptors for Organic Photovoltaic Cells, Y. Kim et al., Polymers, 6, 382-407 (2014).
