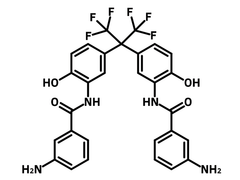
2,2-Bis(3-(3-aminobenzoylamino)-4-hydroxyphenyl)hexafluoropropane (m-6FDAP)
CAS Number 220426-92-6
Chemistry Building Blocks, COF Ligands, Diamines and Dianhydrides, Fluorinated Building Blocks, Materials, Monomers,A fluorinated diamine intermediate
For the preparation of polyimides in application of membranes, capacitors, and photonics
Specifications | MSDS | Literature and Reviews
2,2-Bis(3-(3-aminobenzoylamino)-4-hydroxyphenyl)hexafluoropropane (m-6FDAP), CAS number 220426-92-6, derived from hexafluorodiphenylpropane, is end-capped by two anilines with two pedant hydroxy groups. The amide linkers between the hexafluorodiphenylpropane and the anilines undergo a thermal rearrangement with the adjacent hydroxy groups to form benzoxazoles at 425 °C in nitrogen. The polyimides based on 2,2-Bis(3-(3-aminobenzoylamino)-4-hydroxyphenyl)hexafluoropropane become more rigid and thermally stable and they are used for water permeable membranes.
A core-shell nanocomposite of BaTiO3 and m-6FDAP based polyimides exhibits a discharge energy of 23.1 J/cm3 and charge-discharge efficiency of 65% with promising application in capacitors for energy storage.
The pendant hydroxy groups allow lateral functionalization through Mitsunobu reaction to synthesize grafted polyimides. The quinoline grafted polyimides show nonlinear optical performances with second-harmonic generation coefficient of 15 pm/V (at 635 nm) and electro-optic coefficient of 6 pm/V (at 1300 nm).
Multiple functional groups
For facile synthesis
Fluorinated diamine building block
For photonics, COFs, and polyamides
Worldwide shipping
Quick and reliable shipping
High purity
>99% High purity
General Information
| CAS Number | 220426-92-6 |
| Chemical Formula | C29H22F6N4O4 |
| Full Name | 3,3'-Diamino-N,N'-[perfluoropropane-2,2-diylbis(6-hydroxy-3,1-phenylene)]dibenzamide |
| Molecular Weight | 604.51 g/mol |
| Synonyms | N,N'-[(Perfluoropropane-2,2-diyl)bis(6-hydroxy-3,1-phenylene)]bis(3-aminobenzamide), N,N'- [[2,2,2-Trifluoro-1-(trifluoromethyl)ethylidene]bis(6-hydroxy-3,1-phenylene)]bis[3-aminobenzamide] |
| Classification / Family | Fluorinated building block, Diamine building block, Polyimidies, Membranes, Capacitors, Photonics |
Chemical Structure
Product Details
| Purity | >99% |
| Melting Point | Not Known |
| Appearance | White to pale yellow powder/crystal |
MSDS Documentation
 2,2-Bis(3-(3-aminobenzoylamino)-4-hydroxyphenyl)hexafluoropropane (m-6FDAP) MSDS Sheet
2,2-Bis(3-(3-aminobenzoylamino)-4-hydroxyphenyl)hexafluoropropane (m-6FDAP) MSDS Sheet
Literature and Reviews
-
Equilibrium shift, poisoning prevention, and selectivity enhancement in catalysis via dehydration of polymeric membranes, M.-H. Hyeon et al., Nat Commun., 14, 1673(2023); DOI: 10.1038/s41467-023-37298-y.
-
Interface-tailored relaxor ferroelectric nanocomposites with ultrahigh-insulation shell of fluorinated aromatic polythiourea for high-capacitance energy storage applications, Y. Chen et al., Adv. Electron. Mater., 8, 2200670(2022); DOI: 10.1002/aelm.202200670.
-
Synthesis and characterization of nonlinear optical polymers having quinoline-based chromophores, M. Kim et al, Bull. Korean Chem. Soc., 23(7), 964-970(2002); DOI: 10.5012/bkcs.2002.23.7.964.
