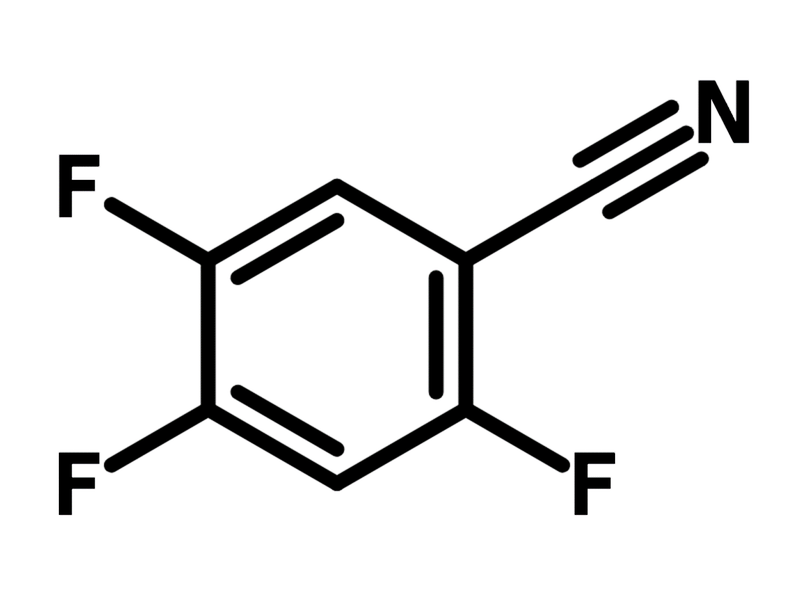
2,4,5-Trifluorobenzonitrile
CAS Number 98349-22-5
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA polyfluorinated benzonitrile building block
Used as a precursor for synthesising APIs and COFs
Specifications | MSDS | Literature and Reviews
2,4,5-Trifluorobenzonitrile (CAS number 98349-22-5) has three fluorides substituting on the benzonitrile core. 2,4,5-Trifluorobenzonitrile serves as a building block for introducing a fluorinated benzonitrile segment to the target molecule by nucleophilic aromatic substitution. A fluorinated analogue of platensimycin, an antibiotic, can be readily synthesised from 2,4,5-trifluorobenzonitrile resulting in a overall yield of 23% over eight steps.
2,4,5-Trifluorobenzonitrile is also employed in the synthesis of dinitrile monomers for polytriazine covalent organic frameworks (COFs). These COFs exhibit BET surface area of 733 m2/g, along with a gas selectivity (CO2/N2) of 48.7 ± 1.2 at 272 K.
Multiple functional groups
For facile synthesis
Fluorinated benzonitrile building block
For triazine COFs, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 98349-22-5 |
| Chemical Formula | C7H2F3N |
| Full Name | 2,4,5-Trifluorobenzonitrile |
| Molecular Weight | 157.10 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building blocks, Benzonitrile building blocks, APIs, COFs, Triazines |
Chemical Structure
Product Details
| Purity | 98% |
| Boiling Point | Tb = 170 °C at 760 mmHg |
| Relative Density | 1.373 g/mL at 25 °C |
| Appearance | Colourless liquid |
MSDS Documentation
 2,4,5-Trifluorobenzonitrile MSDS Sheet
2,4,5-Trifluorobenzonitrile MSDS Sheet
Literature and Reviews
- Structure-activity relationship studies leading to the identification of (2E)-3-[l-[(2,4-dichlorophenyl)methyl]-5-fluoro-3-methyl-lH-indol-7-yl]-N-[(4,5-dichloro-2-thienyl)sulfonyl]-2-propenamide (DG-041) a potent and selective prostanoid EP3 receptor antagonist, as a novel anti-platelet agent that does not prolong bleeding, J. Singh et al., J. Med. Chem.,53(1), 18–36(2010); DOI: 10.1021/jm9005912.
- Semisynthesis and biological evaluation of platensimycin analogues with varying aminobenzoic acid, K. Tian et al., ChemistrySelect., 3(44), 12625–12629; DOI: 10.1002/slct.201802475.
- Evaluation of structure-active relationship of microtubule(MT)-targeting 1,2,4-triazolo[1,5-a]pyrimidines identifies new candidates for neurodegenerative tauopathies, K. Oukoloff et al., J. Med. Chem., 64(2), 1073–1102(2021)DOI: 10.1021/acs.jmedchem.0c01605.
