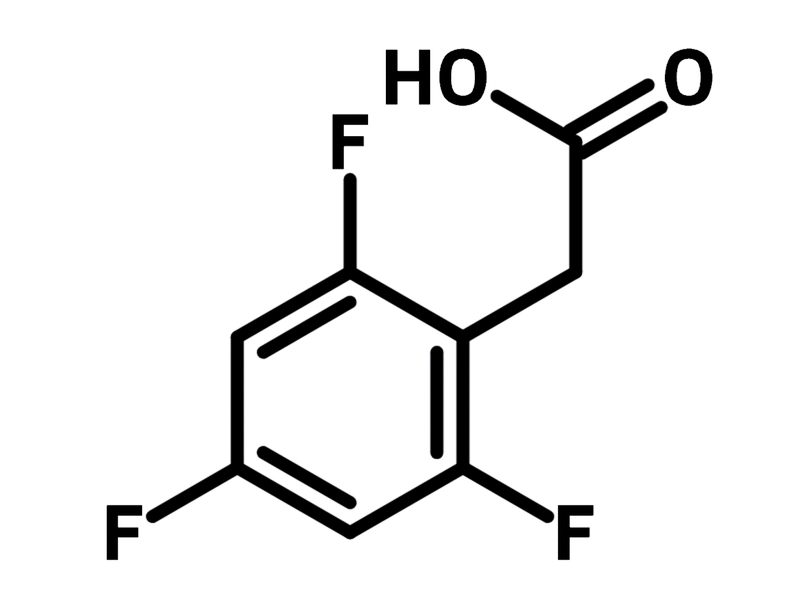
2,4,6-Trifluorophenylacetic acid
CAS Number 209991-63-9
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA trifluorinated phenylacetic acid building block
Used as an intermediate for the preparation of APIs and agricultural fungicides
Specifications | MSDS | Literature and Reviews
2,4,6-Trifluorophenylacetic acid (CAS number 209991-63-9) is a derivative of phenylacetic acid with fluorine substitutions at 2-, 4- and 6-positions. 2,4,6-Trifluorophenylacetic acid readily couples with amines, catalysed by N-hydroxysuccinimide and N,N'-diisopropylcarbodiimide. Glucosamine-based supramolecular nanotubes is prepared with this method, demonstrating effective human mesenchylmal cell therapy.
Additionally, 2,4,6-trifluorophenylacetic acid is employed in the synthesis of pyridazines for agricultural fungicides. The preparation begins with a reaction of 2,4,6-trifluorophenylacetic acid and phenylglyoxylic acid under basic condition to form a furandione derivative. The fungicide is then obtained from a reaction of the resulting furandione and hydrazine.
Multiple functional groups
For facile synthesis
Fluorinated phenylacetic acid building block
for drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 209991-63-9 |
| Chemical Formula | C8H5F3O2 |
| Full Name | 2,4,6-Trifluorophenylacetic acid |
| Molecular Weight | 190.12 g/mol |
| Synonyms | 2,4,6-Trifluorobenzeneacetic acid |
| Classification / Family | Fluorinated building blocks, Phenylacetic acid building blocks, APIs, Fungicides, Heterocycles |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 110 °C – 112 °C |
| Appearance | White crystals |
MSDS Documentation
 2,4,6-Trifluorophenylacetic acid MSDS Sheet
2,4,6-Trifluorophenylacetic acid MSDS Sheet
Literature and Reviews
- Discovery of pyridachlometyl: A new class of pyridazine fungicides, A. Manabe et al., Bioorg. Med. Chem., 88, 117332(2023); DOI: 10.1016/j.bmc.2023.117332.
- Glucosamine-based supramolecular nanotubes for human mesenchymal cell therapy, S. Talloj, ACS Appl. Mater. Interfaces, 10(17), 15079–15087(2018); DOI: 10.1021/acsami.8b03226.
- Atogepant, P. Martelletti et al., Drugs Future, 45(5), 1–13(2020); DOI: 10.1358/dof.2020.45.5.3123467.
