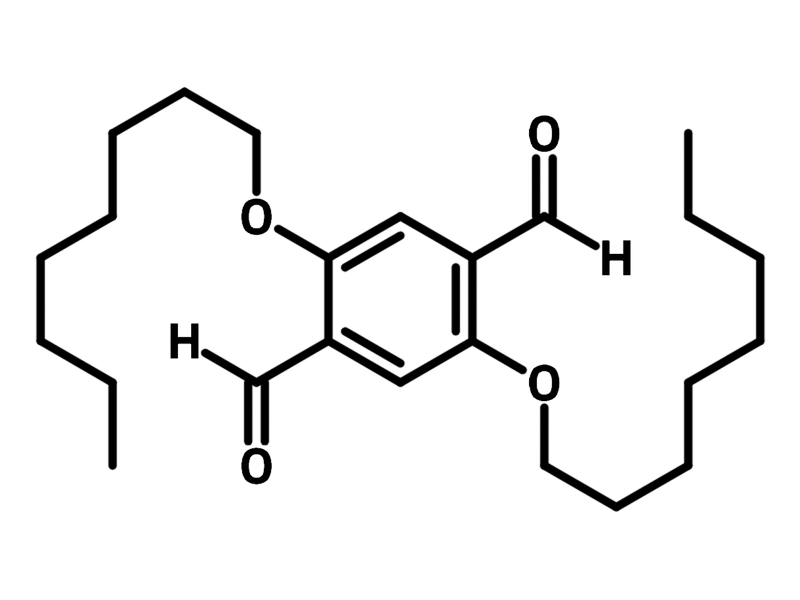
2,5-Bis(octyloxy)terephthalaldehyde
CAS Number 123440-34-6
Carbaldehyde Monomers, Chemistry Building Blocks, COF Ligands, Monomers, Porous Organic FrameworksCovalent Organic Frameworks (COFs) Terephthalaldehyde Ligand
A dialdehyde ligand for the synthesis of COFs and semiconductors in applications of OLEDs, solar cells, and memristors.
Overview | Product Information | Related Products
2,5-Bis(octyloxy)terephthalaldehyde (CAS number 123440-34-6) is a benzene building block with two aldehydes and two n-octyloxy groups. 2,5-Bis(octyloxy)terephthalaldehyde is commonly used for synthesizing covalent organic frameworks (COFs) with amine derivatives such as triaminophenylbenzene. Through the imination reaction of amine and aldehyde, the resulting COF exhibits BET surface area of 1202 m2/g. These porous COFs are applied in memristors with an on/off ratio of an order of magnitude.
In addition to COFs, 2,5-bis(octyloxy)terephthalaldehyde is a synthesis intermediate for vinylene-bridged macromolecules. Such macromolecules have extended π-conjugation, enhancing the charge carrier mobility of organic semiconductors. The fully conjugated backbone allows the resulting polyimine to be used in photovoltaic with power conversion efficiency of 0.17% (preliminary study).
MOF and COF ligands
Aldehyde ligand for cross-linked COF/MOF networks
Facile reactions
Aldehyde possesses excellent reactivity
High purity
> 98% pure
Worldwide shipping
Quick and reliable shipping
General Information
| CAS Number | 123440-34-6 |
|---|---|
| Chemical Formula | C24H38O4 |
| Full Name | 2,5-Bis(octyloxy)terephthalaldehyde |
| Molecular Weight | 390.56 g/mol |
| Synonyms | 2,5-dioctyloxybenzene-1,4-dicarbaldehyde, C8PDA |
| Classification or Family | Carbaldehyde monomers, Alkylated monomers, COFs, Porous organic frameworks |
Chemical Structure
Product Details
| Purity | > 98% |
|---|---|
| Melting Point | 76 – 79 °C |
| Appearance | Bright yellow powder |
MSDS Documentation
2,5-Bis(octyloxy)terephthalaldehyde MSDS Sheet
References
- Effect of functional groups on microporous polymer based resistance switching memory devices, Y. Song et al., Chem. Commun., 56, 6356–6359 (2020); DOI: 10.1039/D0CC01397H
- Novel vinylene-bridged donor–acceptor copolymers: synthesis, characterization, properties and effect of cyano substitution, C. Wei et al., Mater. Chem. Front., 1, 2103 (2017); DOI: 10.1039/c7qm00236j.
- Understanding degradation dynamics of azomethine-containing conjugated polymers, A. Charland-Martin et al., Macromolecules, 57, 6145–6155 (2024); DOI: 10.1021/acs.macromol.4c01168.
Related Products
We stock a wide range of Porous Organic Frameworks available to purchase online. Please contact us if you cannot find what you are looking for.