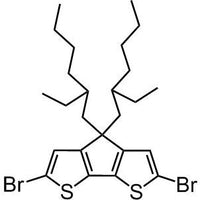
2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene
CAS Number 365547-21-3
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, MonomersHigh-quality monomer that allows more effective conjugation
High-quality and expert support available online
Specifications | MSDS | Literature and Reviews
2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene, CAS number 365547-21-3, with two thiophene units confined to one plane, cyclopenta[2,1-b;3,4-b′]dithiophene compounds allow more effective conjugation if embedded into semiconducting polymers which in turn will have a lower band gap. Furthermore, the planar structure will have greater effect on the morphology for the thin film devices to allow a well packed polymer to polymer backbones which will bring the third-dimension into play. Take PCPDTBT for example, the planar structure improves the hole mobility of the device to 1 x 10-3 cm2/Vs.
2,6-dibromo-4,4-bis(2-ethylhexyl)-4Hcyclopenta[1,2-b:5,4-b']dithiophene can be targeted by reacting 4,4-bis(2-ethylhexyl)-4Hcyclopenta[1,2-b:5,4-b']dithiophene with N-Bromosuccinimide in dimethylformamide.
![synthesis of 2,6-dibromo-4,4-bis(2-ethylhexyl)-4Hcyclopenta[1,2-b:5,4-b']dithiophene using 4,4-bis(2-ethylhexyl)-4Hcyclopenta[1,2-b:5,4-b']dithiophene](https://www.ossila.com/cdn/shop/files/dibromo-bisethylhexyl-cyclopentadithiophene-synthesis.jpg?width=480&height=182)
Capped with bromide
A PCPDTBT monomer
Dithiophene building block
For semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 365547-21-3 |
| Chemical Formula | C25H36Br2S2 |
| Molecular Weight | 560.49 g/mol |
| Synonyms | 2,6-Dibromo-4,4-bis(2-ethylhexyl)-4H-thieno[3',2':4,5]cyclopenta[1,2-b]thiophene 2,6-Dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b']dithiophene |
| Classification / Family | Dithiophene, Cyclopentadithiophene, Organic semiconducting materials, Semiconductor synthesis, Building blocks |
Chemical Structure
Product Details
| Purity | >98% |
| Melting Point | N/A |
| Appearance | Yellow oil |
NMR Characterisation
![1 H NMR spectrum of 2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene in CDCl3](https://www.ossila.com/cdn/shop/files/Dibromo-Bisethylhexylcyclopentabithiophene.png?width=848&height=388)
MSDS Documentation
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene MSDS](https://www.ossila.com/cdn/shop/files/msds-sheets.jpg?width=60&height=60) 2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene MSDS Sheet
2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene MSDS Sheet
Literature and Reviews
- Cyclopentadithiophene-benzothiadiazole oligomers and polymers; synthesis, characterisation, field-effect transistor and photovoltaic characteristics, M. Horie et al., J. Mater. Chem., 22, 381 (2012)
- Copolymers of Cyclopentadithiophene and Electron-Deficient Aromatic Units Designed for Photovoltaic Applications, J.C. Bijleveld et al., Adv. Funct. Mater., 19, 3262-3270 (2009)
- Synthesis and Characterization of Bridged Bithiophene-Based Conjugated Polymers for Photovoltaic Applications: Acceptor Strength and Ternary Blends, C.-H. Chen, Macromolecules, 43 (2), 697-708 (2010)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene CAS 365547-21-3](http://www.ossila.com/cdn/shop/files/4Hcyclopentadithiophene.jpg?v=1718718830&width=240)