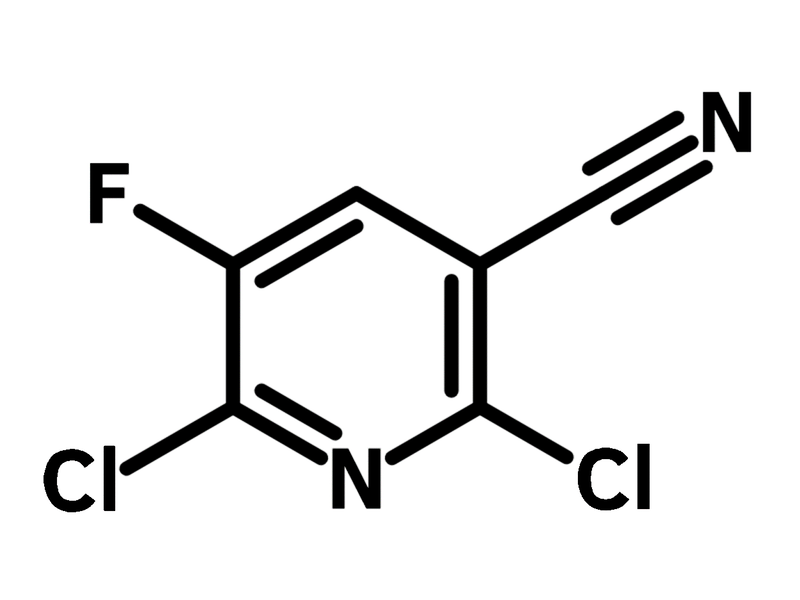
2,6-Dichloro-5-fluoro-3-pyridinecarbonitrile
CAS Number 82671-02-1
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA halogenated pyridinecarbonitrile
Used as a synthetic precursor for APIs
Specifications | MSDS | Literature and Reviews
2,6-Dichloro-5-fluoro-3-pyridinecarbonitrile (CAS number 82671-02-1), also referred to as 2,6-dichloro-3-cyano-5-fluoropyridine, is derived from pyridine with two chlorides, a fluoride and a nitrile substituents. The most recognised reaction using 2,6-dichloro-5-fluoro-3-pyridinecarbonitrile is the synthesis of Gemifloxacin, an oral broad-spectrum antibiotic. Other than Gemifloxacin, 2,6-dichloro-5-fluoro-3-pyridinecarbonitrile is also a molecular scaffold for 1,8-naphthyridine derivatives including Enoxacin, Trovafloxacin and Tosufloxacin. 2,6-Dichloro-5-fluoro-3-pyridinecarbonitrile enables facile synthesis of a wide range of fluoroquinolone derivatives with different building blocks for reducing the side effects of medication and expanding the medical applications.
Multiple functional groups
For facile synthesis
Fluorinated nicotinonitrile building block
For organic synthesis, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 82671-02-1 |
| Chemical Formula | C6HCl2FN2 |
| Full Name | 2,6-Dichloro-5-fluoro-3-pyridinecarbonitrile |
| Molecular Weight | 190.99 g/mol |
| Synonyms | 2,6-Dichloro-3-cyano-5-fluoropyridine, 2,6-Dichloro-5-fluoro-3-nicotinonitrile, 2,6-Dichloro-5-fluoronicotinonitrile |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, APIs, Antibiotics, Bicyclic compounds |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 89 °C – 91 °C |
| Appearance | White powder |
MSDS Documentation
 2,6-Dichloro-5-fluoro-3-pyridinecarbonitrile MSDS Sheet
2,6-Dichloro-5-fluoro-3-pyridinecarbonitrile MSDS Sheet
Literature and Reviews
- Novel fluoroquinolone antibacterial agents containing oxime-substituted (aminomethyl)pyrrolidines: synthesis and antibacterial activity of 7-(4-(aminomethyl)-3-(methoxyimino)pyrrolidin-1-yl)-1-cyclopropyl-6- fluoro-4-oxo-1,4-dihydro[1,8]naphthyridine-3-carboxylic acid (LB20304), C. Hong et al., J. Med. Chem., 40(22), 3584–3593(1997); DOI: 10.1021/jm970202e.
- Synthesis and in vitro antibacterial activity of Gemifloxacin derivatives containing a substituted benzyloxime moiety, L. Feng et al., Eur. J. Med. Chem., 55, 125–136(2012); DOI: 10.1016/j.ejmech.2012.07.010.
- Synthesis, spectroscopic, and biological studies of mixed ligand complexes of Gemifloxacin and glycine with Zn(II), Sn(II), and Ce(III), Molecules, 23, 1182(2018); DOI: 10.3390/molecules23051182.
