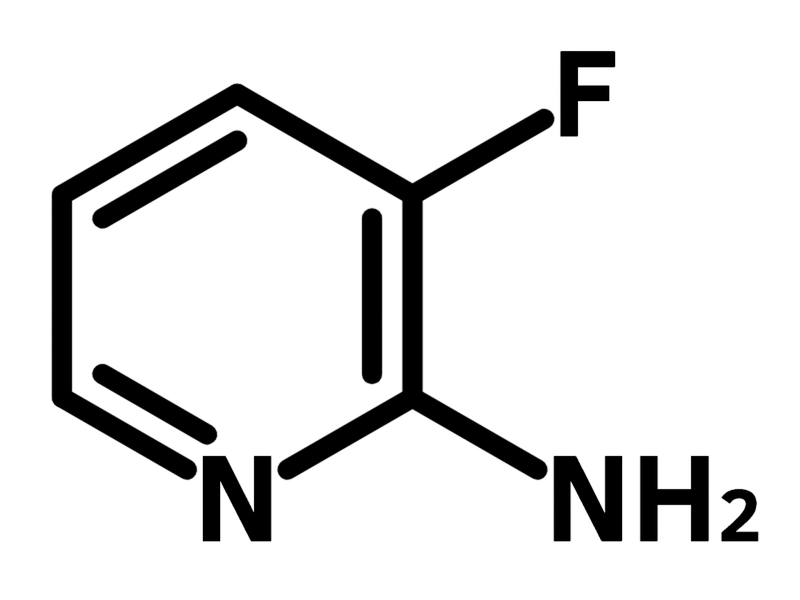
2-Amino-3-fluoropyridine
CAS Number 21717-95-3
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated aminopyridine building block
Used as a precursor for bicyclic heterocycles and a building block for introducing a fluorinated pyridine moiety
Specifications | MSDS | Literature and Reviews
2-Amino-3-fluoropyridine (CAS number 21717-95-3) is derived from pyridine having an amine and a fluorine at 2- and 3-positions. 2-Amino-3-fluoropyridine can be readily introduced to molecular scaffolds by reductive amination and nucleophilic substitution. The modification by 2-amino-3-fluoropyridine has shown a significant enhancement in antitumor activity, compared with the mother molecules. It is suggested that the 2-amino-3-fluoropyridine moiety forms a π–π interaction with the arginine residue in the protein sequence, leading to improved inhibitory.
Furthermore, 2-amino-3-fluoropyridine is also used to synthesise bicyclic heterocycles such as imidazopyridines for pharmaceutical active ingredients through Baylis-Hillman reaction.
Multiple functional groups
For facile synthesis
Fluorinated pyridine building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 21717-95-3 |
| Chemical Formula | C5H5FN2 |
| Full Name | 2-Amino-3-fluoropyridine |
| Molecular Weight | 112.11 g/mol |
| Synonyms | 3-Fluoro-2-pyridinamine |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, APIs, Bicyclic heterocycles |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 41 °C – 45 °C |
| Appearance | White powder/chunks |
MSDS Documentation
 2-Amino-3-fluoropyridine MSDS Sheet
2-Amino-3-fluoropyridine MSDS Sheet
Literature and Reviews
- Multicomponent approach for the synthesis of substituted 1,8-naphthyridine derivatives catalyzed by N-bromosulfonamides, R. Ghorbani-Vaghei et al., Synthesis, 49(04), 763–769(2017); DOI: 10.1055/s-0036-1588886.
- Identification of N-methyl nicotinamide and N-methyl pyridazine-3-carboxamide pseudokinase domain ligands as highly selective allosteric inhibitors of tyrosine kinase 2 (TYK2), R. Moslin et al., J. Med. Chem., 62(20), 8953–8972(2019); DOI: 10.1021/acs.jmedchem.9b00443.
- Studies on condensed-heterocyclic azolium cephalosporins I. Synthesis and antibacterial activity of 7β-[2-(2-aminothiazol-4-yl)-2(Z)-alkoxyiminoacetamido]-3-(imidazo[1,2-a]pyridinium-1-yl)methyl-3-cephem-4-carboxylates, T. Nishimura et al., J. Antibiot.,44(12), 1371–1393(1991); DOI: 10.7164/antibiotics.44.1371.
