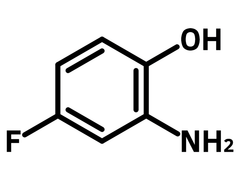
2-Amino-4-fluorophenol
CAS Number 399-97-3
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated aminophenol building block
A precursor for synthesizing benzoxazole and benzoxazine derivatives in application of APIs and fluorescent dyes
Specifications | MSDS | Literature and Reviews
2-Amino-4-fluorophenol (CAS number 399-97-3), also referred to as 5-fluoro-2-hydroxyaniline, has a primary amine and a fluorine substituent at 2- and 4-positions. The amine and hydroxy groups in 2-amino-4-fluorophenol can coordinate to a metal centre to form 5-membered ring complexes. A heteroleptic tin(IV) complex with 2-amino-4-fluorophenol has shown in vitro cytotoxicity towards human cancer cells.
The ortho-position amine and hydroxyl groups make 2-amino-4-fluorophenol an ideal precursor for the syntheses of benzoxazoles and benzoxazines. A fluorescent benzoxazine derivative is synthesized from 2-amino-4-fluorophenol and 1,2-diketones. Functionalised benzoxazole can be obtained by reacting 2-amino-4-fluorophenol with 1,1'-dibromoethene derivatives.
Multiple functional groups
For facile synthesis
Fluorinated aniline/phenol building block
For drug discovery, fluorescent dyes, and organic synthesis
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 399-97-3 |
| Chemical Formula | C6H6FNO |
| Full Name | 2-Amino-4-fluorophenol |
| Molecular Weight | 127.12 g/mol |
| Synonyms | 5-Fluoro-2-hydroxyaniline |
| Classification / Family | Fluorinated building block, APIs and fluorescent dyes |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 130 – 135 °C |
| Appearance | Brown powder |
MSDS Documentation
 2-Amino-4-fluorophenol MSDS Sheet
2-Amino-4-fluorophenol MSDS Sheet
Literature and Reviews
-
4-Benzoyl-2-(4-bromophenyl)-1-(4-methoxyphenyl)-1,2-dihydropyrimidino[4,3-c][1,4]benzoxazine-3,5-dione, E. Khramtsova et al., Molbank, 2023, M1583(2023); DOI:10.3390/M1583.
-
Direct access to highly functionalised benzimidazoles and benzoxazoles from a common precursor, A. Garrido et al., Synthesis, 51, 4006–4013(2019); DOI: 10.1055/s-0039-1690153.
-
Structure-based optimization of small molecule human galactokinase inhibitors, L. Liu et al., J. Med. Chem., 64(18), 13551–13571(2021); DOI: 10.1021/acs.jmedchem.1c00945.
