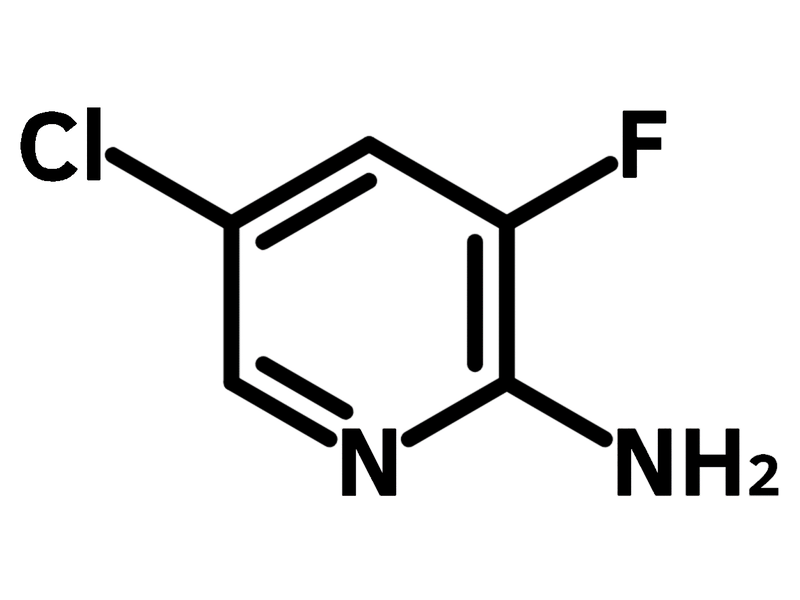
2-Amino-5-chloro-3-fluoropyridine
CAS Number 246847-98-3
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated aminopyridine building block
For the synthesis of APIs and metal complexes
Specifications | MSDS | Literature and Reviews
2-Amino-5-chloro-3-fluoropyridine (CAS number 246847-98-3) is a pyridine derivative substituted with an amine, a fluorine and a chlorine at the 2-, 3- and 5-positions respectively. The pyridinium amine of 2-amino-5-chloro-3-fluoropyridine can easily coordinate to copper centres, in neutral or acidic media. The resulting organometallic complexes exhibit antiferromagnetic interactions under temperatures ranging from −0.9 to −31.3 K.
2-Amino-5-chloro-3-fluoropyridine is also used in the synthesis of active pharmaceutical ingredients (APIs). These APIs include inhibitors of cyclin-de-pendent kinase for cancer treatments and disruptors of glucokinase-glucokinase regulatory protein for type II diabetes mellitus.
Multiple functional groups
For facile synthesis
Fluorinated aminopyridine building block
For drug discovery, medicinal chemistry, and organometallic complexes
Low Cost
Competitively priced, high quality product
High purity
>97% High purity
General Information
| CAS Number | 246847-98-3 |
| Chemical Formula | C5H4ClFN2 |
| Full Name | 5-Chloro-3-fluoro-2-pyridinamine |
| Molecular Weight | 146.55 g/mol |
| Synonyms | 5-Chloro-3-fluoropyridin-2-amine, 5-chloro-3-fluoro-2-pyridylamine |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Pyridine building blocks, Amine building blocks, Organometallic complexes, APIs |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 91 °C – 95 °C |
| Appearance | Beige powder |
MSDS Documentation
 2-Amino-5-chloro-3-fluoropyridine MSDS Sheet
2-Amino-5-chloro-3-fluoropyridine MSDS Sheet
Literature and Reviews
- Copper(II) complexes of 2-amino-5-chloro-3-fluoropyridine: syntheses and magnetic properties of (3,5-FCAP)2CuX2 and (3,5-FCAPH)2CuX4, B. Solomon et al., J. Coord. Chem., 67, 3953–3971 (2014); DOI: 10.1080/00958972.2014.893431.
- Discovery of potent, selective, and orally bioavailable small-molecule modulators of the mediator complex-associated kinases CDK8 and CDK19, A. Mallinger et al., J. Med. Chem., 59, 1078–1101 (2016); DOI: 10.1021/acs.jmedchem.5b01685.
- Discovery and structure-guided optimization of diarylmethanesulfonamide disrupters of glucokinase−glucokinase regulatory protein (GK−GKRP) binding: strategic use of a N → S (nN → σ*S−X) interaction for conformational constraint, L. Pennington et al., J. Med. Chem., 58, 9663–9679 (2015); DOI: 10.1021/acs.jmedchem.5b01367.
