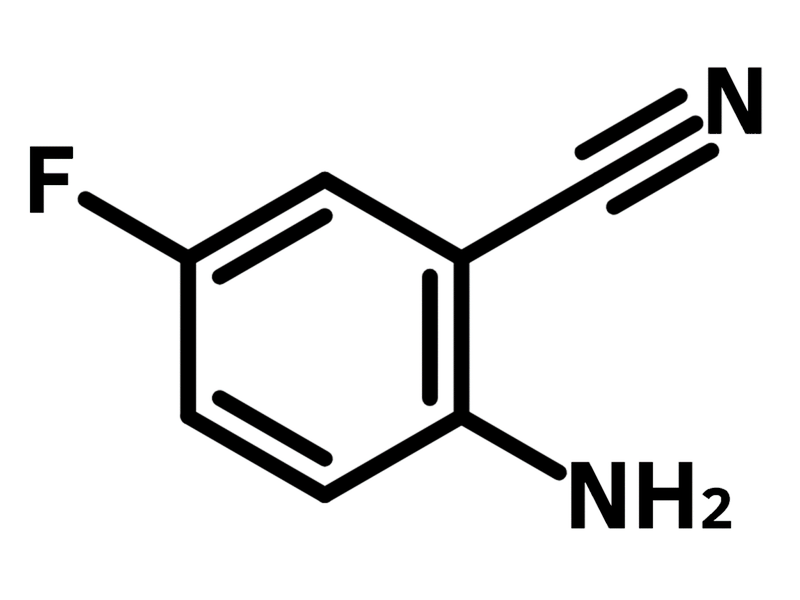
2-Amino-5-fluorobenzonitrile
CAS Number 61272-77-3
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA fluorinated o-aminobenzonitrile
Used as a synthetic precursor for heterocycles in the applications of APIs and ligands
Specifications | MSDS | Literature and Reviews
2-Amino-5-fluorobenzonitrile (CAS number 61272-77-3) possesses multiple functional groups, including an amine, a nitrile and a fluoride. The ortho-positioning of the amine and nitrile substituents makes 2-amino-5-fluorobenzonitrile an ideal precursor for synthesising heterocyclic compounds. 2-Amino-5-fluorobenzonitrile reacts with aminoethanol derivatives catalysed by zinc chloride, yielding oxazoline ligands for use in Cu-catalysed enantioselective nitroaldol reactions. Through its reaction with cyclic ketone in Friedländer reaction, 2-amino-5-fluorobenzonitrile serves as a precursor for tacrine derivatives. In the case of synthesising quinazoline/quinazolinone derivatives, the reaction of 2-amino-5-fluorobenzonitrile with cyclic ketones derivatives occurs under basic condition.
Multiple functional groups
For facile synthesis
Fluorinated benzonitrile/aniline building block
For organometallic catalysis, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 61272-77-3 |
| Chemical Formula | C7H5FN2 |
| Full Name | 2-Amino-5-fluorobenzonitrile |
| Molecular Weight | 136.13 g/mol |
| Synonyms | 2-Amino-5-fluorobenzenecarbonitrile, 2-Cyano-4-fluoroaniline |
| Classification / Family | Fluorinated building blocks, Aniline building blocks, Benzonitrile building blocks, APIs, Heterocycles, Ligands |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 92 °C – 96 °C |
| Appearance | Grey powder |
MSDS Documentation
 2-Amino-5-fluorobenzonitrile MSDS Sheet
2-Amino-5-fluorobenzonitrile MSDS Sheet
Literature and Reviews
- Structure-activity relationships of dually-acting acetylcholinesterase inhibitors derived from tacrine on N-methyl-D-Aspartate receptors, L. Gorecki et al., Eur. J. Med. Chem., 219, 113434(2021); DOI: 10.1016/j.ejmech.2021.113434.
- Cobalt-catalyzed tandem transformation of 2-aminobenzonitriles to quinazolinones using hydration and dehydrogenative coupling strategy, A. Samin et al., J. Org. Chem., 85, 11359–11367(2020); DOI: 10.1021/acs.joc.0c01307.
- Chiral pyridine oxazoline and 1,2,4‑triazine oxazoline ligands incorporating electron‑withdrawing substituents and their application in the Cu‑catalyzed enantioselective nitroaldol reaction, E. Wolińska et al., Monatsh. Chem., 153, 245–256(2022); DOI: 10.1007/s00706-022-02893-0.
