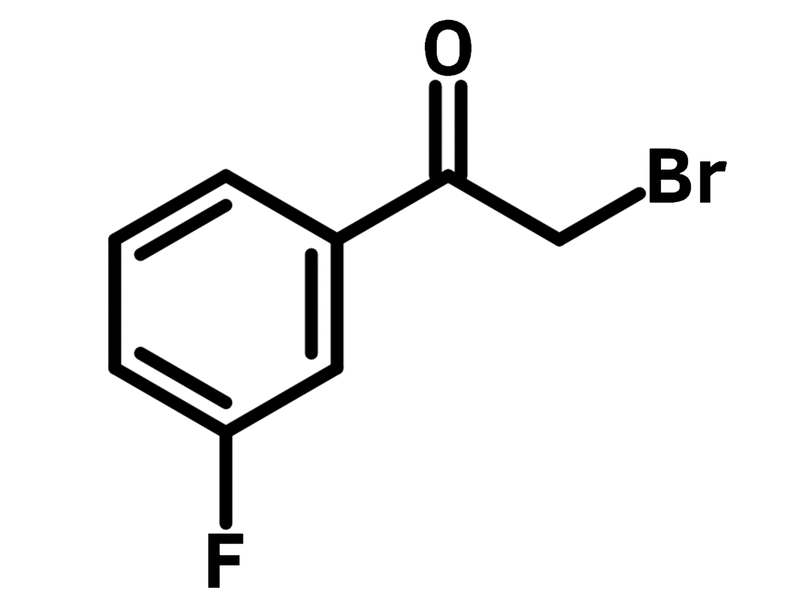
2-Bromo-3′-fluoroacetophenone
CAS Number 53631-18-8
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA dihalogenated acetophenone building block
Used as a precursor for heterocycles and ligands for APIs and phosphorescent complexes
Specifications | MSDS | Literature and Reviews
2-Bromo-3′-fluoroacetophenone (CAS number 53631-18-8), alternatively known as 3-fluorophenylacyl bromide, has a fluorine substituent on the benzene ring and a bromine on the α-carbon of the ketone. 2-Bromo-3′-fluoroacetophenone is popularly used to synthesised heterocycles such as pyrazines and thiazoles. Thiazoles are obtained from 2-bromo-3′-fluoroacetophenone reacting with thiourea in ethanol (reflux). The thiazolyl derivatives are applied as correctors of the chloride transport defect in cystic fibrosis, glutathione S-transferase Omega 1 inhibitors and Trypanosoma brucei, to name a few.
2-Bromo-3′-fluoroacetophenone reacts with ortho-phenylenediamine derivatives to produce pyrazine derivatives. Complexes of iridium-pyrazine derivatives show phosphorescent emitting yellow to deep red colours (λem = 579 – 655 nm).
Multiple functional groups
For facile synthesis
Fluorinated acetophenone building block
For phosphorescent complexes, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 53631-18-8 |
| Chemical Formula | C8H6BrFO |
| Full Name | 2-Bromo-1-(3-fluorophenyl)ethan-1-one |
| Molecular Weight | 217.04 g/mol |
| Synonyms | 3-Fluorophenacyl bromide, 2-Bromo-1-(3-fluorophenyl)ethanone |
| Classification / Family | Fluorinated building blocks, Heterocycles, Phosphorescent complexes, APIs |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 33 °C – 37 °C |
| Appearance | White crystals |
MSDS Documentation
 2-Bromo-3′-fluoroacetophenone MSDS Sheet
2-Bromo-3′-fluoroacetophenone MSDS Sheet
Literature and Reviews
- Polysubstituted ligand framework for color tuning phosphorescent iridium(III) complexes, S. Fitzgerald et al., Inorg. Chem., 60(20), 15467–15484(2021); DOI: 10.1021/acs.inorgchem.1c02121.
- Structure-based design of N-(50phenylthiazol-2-yl)acrylamides as novel and potent glutathione S-transferase omega 1 inhibitors, W. Dai et al., J. Med. Chem., 62(6), 3068–3087(2019); DOI: 10.1021/acs.jmedchem.8b01960.
- Synthesis and structure-activity relationship of aminoarylthiazole derivatives as correctors of the chloride transport defect in cystic fibrosis, E. Pesce et al., Eur. J. Med. Chem., 99, 14–35(2015); DOI: 10.1016/j.ejmech.2015.05.030.
