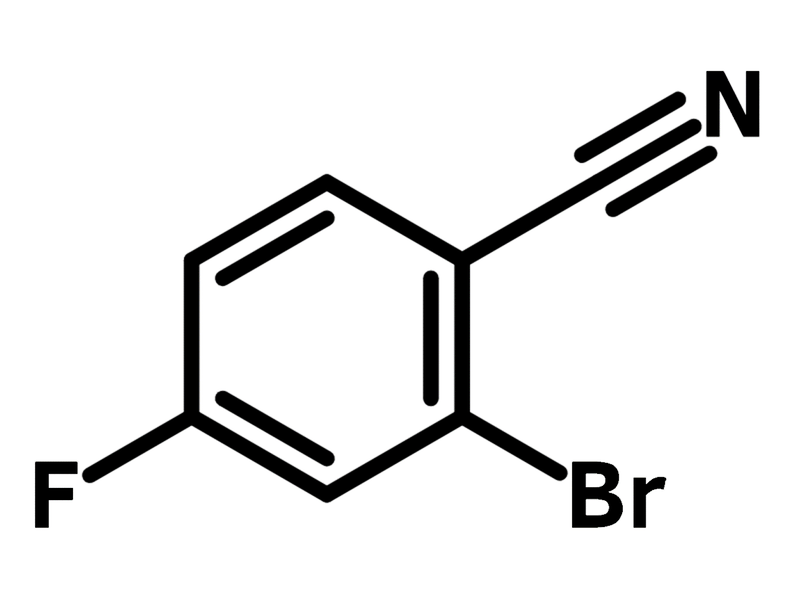
2-Bromo-4-fluorobenzonitrile
CAS Number 36282-26-5
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA dihalogenated benzonitrile building block
Used as a molecular scaffold and building building block for APIs
Specifications | MSDS | Literature and Reviews
2-Bromo-4-fluorobenzonitrile (CAS number 36282-26-5) has a bromide and a fluoride substituents on benzonitrile at ortho- and para-position, respectively. With three different functional groups, 2-bromo-4-fluorobenzonitrile is commonly used as a molecular scaffold for active pharmaceutical ingredients (APIs). The bromo-substituent can undergo Pd catalysed C-C coupling, while the fluoride is an ideal leaving group for nucleophilic aromatic substitution. The nitrile group not only acts as the activator for the nucleophilic substitution, but also can react with hydroxylamine to form oxadiazoles. The oxadiazole derivatives are type 2 cannabinoid receptors used for treatments of central nervous system disorders.
2-Bromo-4-fluorobenzonitrile is also used to introduce reactive functional groups to molecular scaffolds and as a substrate with multiple functional groups in synthetic chemistry.
Multiple functional groups
For facile synthesis
Fluorinated benzonitrile building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 36282-26-5 |
| Chemical Formula | C7H3BrFN |
| Full Name | 2-Bromo-4-fluorobenzonitrile |
| Molecular Weight | 200.01 g/mol |
| Synonyms | 4-Fluoro-2-bromobenzonitrile |
| Classification / Family | Fluorinated building blocks, Benzonitrile building blocks, Heterocycles, APIs |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 77 °C |
| Appearance | Pale purple powder |
MSDS Documentation
 2-Bromo-4-fluorobenzonitrile MSDS Sheet
2-Bromo-4-fluorobenzonitrile MSDS Sheet
Literature and Reviews
- Cannabinoid receptor type 2 (CB2)-selective N-aryl-oxadiazolyl-propionamides: synthesis, radiolabelling, molecular modelling and biological evaluation, T. Rühl et al., Org. Med. Chem. Lett., 2(1), 32(2012); DOI: 10.1186/2191-2858-2-32.
- Highly triplet energy iridium(III) isocyanoborato complex for photochemical up conversion, photoredox and energy transfer catalysis, L. Schmid et al., J. Am. Chem. Soc., 144, 963–976(2022); DOI: 10.1021/jacs.1c11667.
- Hybrid dual aromatase-steroid sulfatase inhibitors with exquisite picomolar inhibitory activity, L. Woo et al., ACS Med. Chem. Lett., 2, 243–247(2011); DOI: 10.1021/ml100273k.
