2-Bromonaphthalene
CAS Number 580-13-2
Chemistry Building Blocks, Materials, Monomers, Non-Heterocyclic Building Blocks2-Bromonaphthalene, a solid additive to polymer solar cells
Useful intermediate for the construction of semiconducting molecules, oligomers in application of OLEDs, OFETs and OPVs.
Specifications | MSDS | Literature and Reviews
2-Bromonaphthalene (CAS number 580-13-2), a mono-brominated naphthalene at 2-position, is normally used as a solid additive to replace 1-chloronaphthalene (1CN) to increase miscibility of the bulk heterojunction (BHJ) components. It is also a widely used intermediate for the construction of more complex structures in application of organic electronic devices.
As an additive to the active layer mixture, 2-bromonaphthalene is employed in small percentages to modify the morphology of the solution processed film thus greatly impact the efficiency of BHJ polymer solar cells. The photovoltaic performance of the PSCs based on the polymer donor of PTB7 and fullerene acceptor PC71BM was optimized using 5 vol% high-boiling-point solvent additive of 2-bromonaphthalene exhibited a power conversion efficiency of 7.01% with open-circuit voltage (VOC) of 0.731 V, short-circuit current density (JSC) of 13.79 mA cm-2, and fill factor (FF) of 69.46% [1].
Also 2-bromonaphthalene derived 2,6-bis(6-hexyloxy-naphthalen-2-yl)anthracene, showed a high mobility of 0.64 cm2 V-1 s-1. The electron-donating decyloxy groups were introduced to the naphthalene moiety not only to tune the electronic properties of the small fused acenes, but also to increase the self-assembly stacking property.
Naphthalene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Substituted with a bromide group
for facile reactions
High purity
>98% Purity
General Information
| CAS Number | 580-13-2 |
| Chemical Formula | C10H7Br |
| Full Name | 2-Bromonaphthalene |
| Molecular Weight | 207.07 g/mol |
| Synonyms | 2-BN |
| Classification / Family | Naphthalenes, Semiconductor synthesis intermediates, Low band gap polymers, OLED, OFETs, organic photovoltaics |
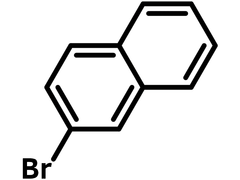
Chemical Structure
Product Details
| Purity | >98% (1H NMR) |
| Melting Point | Tm = 58 °C |
| Appearance | White to orange powder/crystals |
MSDS Documentation
Literature and Reviews
- Effects of high-boiling-point additive 2-Bromonaphthalene on polymer solar cells fabricated in ambient air, X. Sun et al., Polym. Bull. 74, 4515–4524 (2017); DOI: 10.1007/s00289-017-1971-9.
- Organic Semiconductor Based on Asymmetric Naphthalene-Thiophene Molecule for Organic Thin Film Transistor, S. Park et al., J. Nanosci. Nanotechnol., 14 (8), 6172–6176 (2014); DOI: :10.1166/jnn.2014.8805.
- Triphenylamine/Tetracyanobutadiene-Based π-Conjugated Push–Pull Molecules End-Capped with Arene Platforms: Synthesis, Photophysics, and Photovoltaic Response, P. Marqués et al., Chem. Eur. J., 26 (69), 16422-16433 (2020); DOI: 10.1002/chem.202002810.

 2-Bromonaphthalene MSDS Sheet
2-Bromonaphthalene MSDS Sheet