2-Dodecylthiophene
CAS Number 4861-61-4
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, MonomersAn intermediate leading to more complex structures
With the tendency to aggregate and self-aggregate, it gives rise to orderly packed molecules in film.
Specifications | MSDS | Literature and Reviews
2-Decylthiophene (CAS number 4861-61-4) with a dodecyl (C12H25) carbon hydrate side chain, is an useful alkylated thiophene that is commonly used as an intermediate for the construction of semiconducting polymers or small molecules for device fabrication of organic field-effect transistors and polymer solar cells.
PTVTTT2T, a copolymer with a backbone of biaxially 2-dodecylthiophene side chained thieno[3,2-b]thiophene (TT2T) and thienylene-vinylene-thienylene (TVT), possessed better molecular packing in thin films with a nanofiber structure owing to its coplanar backbone and the optimum side chain length for solubility and film morphology. The average field-effect charge mobility of PTVTTT2T was found to be 0.64 cm2 V-1 s-1 with a high on/off ratio over 107 under ambient environment for over three months.
Thiophene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Substituted with a dodecyl group
for improving solubility
High purity
>98% Purity
General Information
| CAS Number | 4861-61-4 |
| Chemical Formula | C16H28S |
| Full Name | 2-Decylthiophene |
| Molecular Weight | 252.46 g/mol |
| Synonyms | N/A |
| Classification / Family | Thiophene, Semiconductor synthesis intermediates, Low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
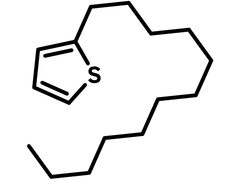
Product Details
| Purity | >98% (1H NMR) |
| Boiling Point | Tm = 155 °C at 2 mmHg |
| Appearance | Light yellow to orange liquid |
| Relative Density | 0.91 |
MSDS Documentation
Literature and Reviews
- Biaxially Extended Conjugated Polymers with Thieno[3,2-b]thiophene Building Block for High Performance Field-Effect Transistor Applications, P. Chao et al., Macromolecules, 48, 16, 5596–5604 (2015); DOI: 10.1021/acs.macromol.5b01243.
- Star-like substituted hexaarylbenzenes: synthesis and mesomorphic properties, Y. Geng et al., J. Mater. Chem., 11, 1634-1641 (2011); DOI: 10.1039/B101163O.
- Effect of lipid on formation of Maillard and lipid-Maillard meaty flavour compounds in heated cysteine-xylose-methyl linoleate system, Y. Wang et al., Flavour Fragr. J., 37 (5), 274-284 (2022); DOI: 10.1002/ffj.3710.


 2-Dodecylthiophene MSDS Sheet
2-Dodecylthiophene MSDS Sheet