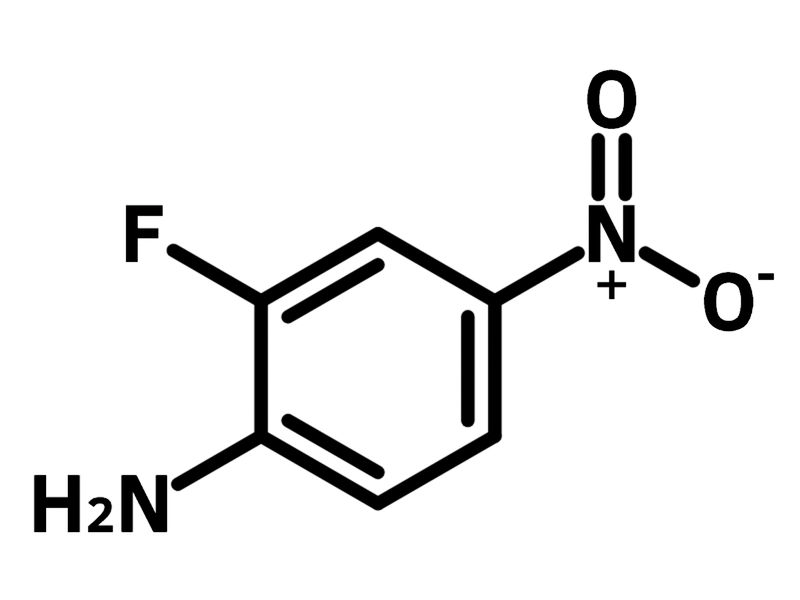
2-Fluoro-4-nitroaniline
CAS Number 369-35-7
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated aniline building block
Used as a synthesis intermediate for APIs, colour centre-tailored carbon nanotubes and anode materials in Li-ion batteries
Specifications | MSDS | Literature and Reviews
2-Fluoro-4-nitroaniline, (CAS number 369-35-7) is an aniline derivative bearing a fluoride and a nitro group at 2- and 4-positions. 2-Fluoro-4-nitroaniline serves as a versatile molecular scaffold for synthesising active pharmaceutical ingredients (APIs). The amine group conducts nucleophilic substitution and diazotization. The nitro group can be laterally reduced to amine for further reactions. A precursor to the antibiotic candidate TBI-233 is synthesised from 2-fluoro-4-nitroaniline.
2-Fluoro-4-nitroaniline was used to introduce sp3 defects onto semiconducting single-walled carbon nanotubes (SWCNTs) for organic colour centre-tailored carbon nanotubes. A lithium bismaleamate anode material is synthesised from 2-fluoro-4-nitroaniline, demonstrating an electrode capacity of 688.9 mAhg−1.
Multiple functional groups
For facile synthesis
Fluorinated aniline building block
For semiconductors, batteries and colour centre-tailored carbon nanotubes
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 369-35-7 |
| Chemical Formula | C6H5FN2O2 |
| Full Name | 2-Fluoro-4-nitroaniline |
| Molecular Weight | 156.11 g/mol |
| Synonyms | 2-Fluoro-4-nitrobenzenamine |
| Classification / Family | Fluorinated building blocks, Aniline building blocks, APIs, Li-ion batteries, Anode materials, Colour centre-tailored carbon nanotubes |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 122 °C – 130 °C |
| Appearance | Brown powder |
MSDS Documentation
 2-Fluoro-4-nitroaniline MSDS Sheet
2-Fluoro-4-nitroaniline MSDS Sheet
Literature and Reviews
- Synthesis, characteristics, and electrochemical performance of N,N-(p-phenylene)bismaleamate and its fluorosubstitution compound on organic anode materials in lithium-ion batteries, B. Kahsay et al., Electrochim. Acta, 365, 137342(2021); 10.1016/j.electacta.2020.137342.
- Practical and scalable two-step process for 6-(2-fluoro-4-nitrophenyl)-2-oxa-6-azaspiro[3.3]heptane: a key intermediate of the potent antibiotic drug candidate TBI-223, F. Cardoso et al., Org. Process Res. Dev., 27, 1390–1399(2023); DOI: 10.1021/acs.oprd.3c00148.
- Discovery of novel conjugates of quinoline and thiazolidinone urea as potential anti-colorectal cancer agent, L. Xiong et al., J. Enzyme Inhib. Med. Chem., 37(1), 2334–2347(2022); DOI: 10.1080/14756366.2022.2117318.
