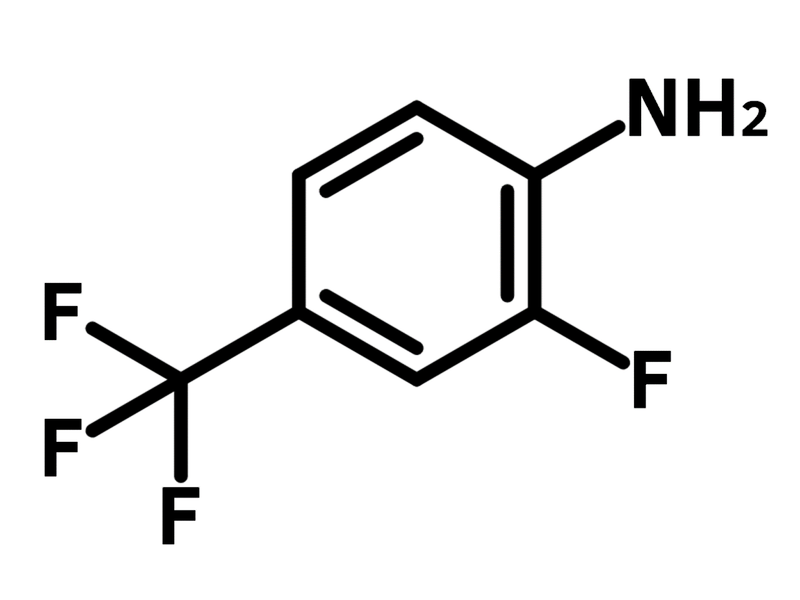
2-Fluoro-4-(trifluoromethyl)aniline
CAS Number 69409-98-9
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA fluorinated aniline building block
Used as a synthesis intermediate for heterocycle derivatives, triarylmethanes and phenylacetamides in application of APIs and dyes
Specifications | MSDS | Literature and Reviews
2-Fluoro-4-(trifluoromethyl)aniline (CAS number 69409-98-9) is an aniline derivate, bearing a fluorine and a trifluoromethyl at 2- and 4-positions. The ortho-substituted fluorine can perform nucleophilic aromatic substitution, making 2-fluoro-4-(trifluoromethyl)aniline an excellent precursor for bicyclic heterocycles, such as quinoxalines and quinoline, as well as tricyclic heterocycles such as benzoimidazotriazines, phenazines and phenoxazines. 2-Fluoro-4-(trifluoromethyl)aniline can be used as a building block for ocfentanil derivatives as an analgesic.
2-Fluoro-4-(trifluoromethyl)aniline is also used to synthesise triarylmethane derivatives with para-quinone methides via conjugated addition.
Multiple functional groups
For facile synthesis
Fluorinated aniline building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 69409-98-9 |
| Chemical Formula | C7H5F4N |
| Full Name | 2-Fluoro-4-(trifluoromethyl)aniline |
| Molecular Weight | 179.11 g/mol |
| Synonyms | 4-Amino-3-fluorobenzotrifluoride, 2-Fluoro-4-(trifluoromethyl)benzenamine |
| Classification / Family | Fluorinated building block, Heterocycles, Triarylmethanes, APIs |
Chemical Structure
Product Details
| Purity | 98% |
| Boiling Point | Tb = 55 °C at 0.3 mmHg |
| Appearance | Colourless liquid |
MSDS Documentation
 2-Fluoro-4-(trifluoromethyl)aniline MSDS Sheet
2-Fluoro-4-(trifluoromethyl)aniline MSDS Sheet
Literature and Reviews
- Synthesis and in vitro evaluation of 8-pyridinyl=substituted benzo[e]imidazo[2,1-c][1,2,4]triazines as phosphodiesterase 2A inhibitors, R. Ritawidya et al., Molecules, 24, 2791(2019); DOI: 10.3390/molecules24152791.
- Silver-catalyzed regioselective 1,6-hydroarylation of para-quinone methides with anilines and phenols, B, Xiong et al., Org. Chem. Front., 9, 3807–3817(2022); DOI: 10.1039/D2QO00541G.
- Synthesis and biological evaluation of 2-(3-fluoro-4-nitrophenoxy)-N-phenylacetamide derivatives as novel potenial affordable antitubercular agents, W. Ang et al., Molecules, 17, 2248–2258; DOI: 10.3390/molecules17022248.
