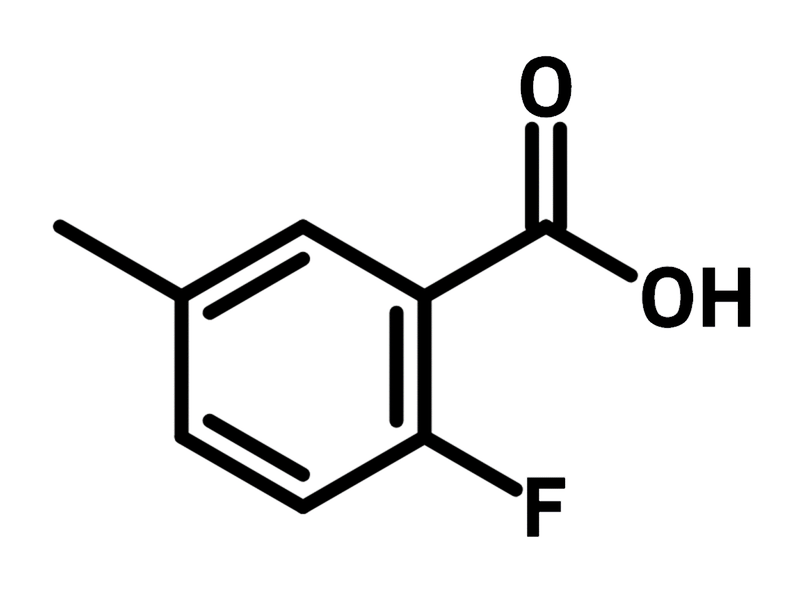
2-Fluoro-5-methylbenzoic acid
CAS Number 321-12-0
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA fluorinated benzoic acid building block
Serves as a synthesis intermediate for APIs
Specifications | MSDS | Literature and Reviews
2-Fluoro-5-methylbenzoic acid (CAS number 321-12-0), also known as 6-fluoro-m-toluic acid, is a meta-toluic acid with a fluoride substituent at the 2-position. The carboxylic acid group enables facile amidation with the use of hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU). Benzoxazepinones, highly potent and monoselective kinase inhibitors, can be obtained from the corresponding 2-fluoro-5-methylbenzoic acid amide derivatives through an intramolecular nucleophilic aromatic substitution reaction.
2-Fluoro-5-methylbenzoic acid is also employed in the synthesis of pyrimidinone derivatives for the selective inhibition of adenylyl cyclase 1 (AC1) in the treatment of chronic pain. The resulting product exhibits an inhibitory concentration against AC1 of 0.54 μM.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
For drug discovery, medicinal chemistry, and biochemistry
Low Cost
Competitively priced, high quality product
High purity
>97% High purity
General Information
| CAS Number | 321-12-0 |
| Chemical Formula | C8H7FO2 |
| Full Name | 2-Fluoro-5-methylbenzoic acid |
| Molecular Weight | 154.14 g/mol |
| Synonyms | 3-Carboxy-4-fluorotoluene, 6-Fluoro-m-toluic acid |
| Classification / Family | Fluorinated building blocks, Benzoic acid building blocks, APIs |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 160 °C – 162 °C |
| Appearance | White powder |
MSDS Documentation
 2-Fluoro-5-methylbenzoic acid MSDS Sheet
2-Fluoro-5-methylbenzoic acid MSDS Sheet
Literature and Reviews
- DNA-encoded library screening identifies benzo[b][1,4]oxazepin-4-ones as highly potent and mono-selective receptor interacting protein 1 (RIP1) kinase inhibitors, P. Harris et al., J. Med. Chem., 59 (5), 2163–2178 (2016); DOI: 10.1021/acs.jmedchem.5b01898.
- Based-promoted chemodivergent formation of 1,4-benzoxazepin-5(4H)-ones and 1,3-benzoxazin-4(4H)-ones switched by solvents, Q. Chen et al., Molecules, 24, 3773 (2019); DOI: 10.3390/molecules24203773.
- Optimization of a pyrimidinone series for selective inhibition of Ca2+/calmodulin-stimulated adenylyl cyclase 1 activity for the treatment of chronic pain, J. Scott et al., J. Med. Chem., 65 (6), 4667–4686 (2022); DOI: 10.1021/acs.jmedchem.1c01759.
