2-Fluoro-5-nitrobenzonitrile
CAS Number 17417-09-3
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated benzene with multiple functional groups
A molecular scaffold for synthesizing APIs and macromolecules
Specifications | MSDS | Literature and Reviews
2-Fluoro-5-nitrobenzonitrile (CAS number 17417-09-3) is a benzene derivative with a fluorine, a nitrile and a nitro electron withdrawing groups. The triple-substituted 2-fluoro-5-nitrobenzonitrile is an ideal scaffold for molecular design. Each substituent has different reactivities, thus no protective group is required. Anti-tumour benzothiophene derivatives can be synthesized from 2-fluoro-5-nitrobenzonitrile reacting with methyl thioglycolate (93% yield). The synthetic route is fully customised and different functional groups can be introduced to the molecular scaffold depending on the desired lipophilicity and binding affinity. Other than benzothiophene, pyrroloquinoline and quinoline derivatives can be synthesized from 2-fluoro-5-nitrobenzonitrile.
The meta-position of nitro and nitrile groups allow 2-fluoro-5-nitrobenzonitrile to construct macrocycles.
Multiple functional groups
For facile synthesis
Fluorinated benzonitrile building block
For drug discovery, organic synthesis and medicinal chemistry
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 17417-09-3 |
| Chemical Formula | C7H3FN2O2 |
| Full Name | 2-Fluoro-5-nitrobenzonitrile |
| Molecular Weight | 166.11 g/mol |
| Synonyms | 3-Cyano-4-fluoronitrobenzene, 5-Nitro-2-fluorobenzonitrile |
| Classification / Family | Fluorinated building block, Molecular scaffold, APIs, Macromolecules, Macrocycles |
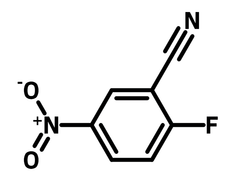
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 76 – 80 °C |
| Appearance | White to off-white powder |
MSDS Documentation
 2-Fluoro-5-nitrobenzonitrile MSDS Sheet
2-Fluoro-5-nitrobenzonitrile MSDS Sheet
Literature and Reviews
-
Concomitant ring contraction cyclization strategy for the synthesis of novel 4-oxo-4,5-dihydro-pyrroloquinolines, G. Manh et al., Tetrahedron Lett., 45, 5913–5916(2004); DOI: 10.1016/j.tetlet.2004.05.1.
-
Microwave-assisted synthesis of 3-aminobenzo[b]-thiophene scaffolds for the preparation of kinase inhibitors, M. Bagley et al., Org. Biomol. Chem., 13, 6814(2015); DOI: 10.1039/c5ob00819k.
-
Structure-based design of macrocyclic coagulation factor VIIa inhibitors, E. Priestley et al., J. Med. Chem., 58(15), 6225–6236(2015); DOI: 10.1021/acs.jmedchem.5b00788.
