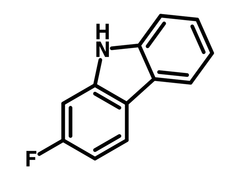
2-Fluoro-9H-carbazole
CAS Number 391-53-7
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated carbazole building block
Used as a synthesis intermediate for bioactive compounds and emitters in application of antimicrobials and OLEDs
Specifications | MSDS | Literature and Reviews
2-Fluoro-9H-carbazole (CAS number 391-53-7), derived from carbazole, is a fluorinated aromatic heterocyclic compound with two benzene rings fused by a pyrrole. As the analogue of carbazole, 2-Fluoro-9H-carbazole and its derivatives show antibacterial performance with minimum inhibitory concentration of 25 µg/mL against E. Coli, B. subtilis and S. aureus. 2-Fluoro-9H-carbazole can be easily attached to another halogenated building block via Buchwald-Hartwig amination and copper catalysed alkylation, to enlarge the chromophore size of the molecules.
A fluorinated polyvinylcarbazole synthesised from 2-fluoro-9H-carbazole is exploited as a host material in organic light emitting diodes. It has a higher luminous current efficiency of 24 cd/A than polyvinylcarbazole (17 cd/A), owing to the wider HOMO-LUMO energy gap of the fluorinated polymers.
Multiple functional groups
For facile synthesis
Fluorinated carbazole building block
For drug discovery, organic synthesis, and OLEDs
Worldwide shipping
Quick and reliable shipping
High purity
>99% High purity
General Information
| CAS Number | 391-53-7 |
| Chemical Formula | C12H8FN |
| Full Name | 2-Fluoro-9H-carbazole |
| Molecular Weight | 185.20 g/mol |
| Synonyms | 2-Fluorocarbazole |
| Classification / Family | Fluorinated building block, Heterocyclic building block, OLEDs, APIs |
Chemical Structure
Product Details
| Purity | 99% |
| Melting Point | Tm = 227 °C |
| Appearance | Off-white powder |
MSDS Documentation
 2-Fluoro-9H-carbazole MSDS Sheet
2-Fluoro-9H-carbazole MSDS Sheet
Literature and Reviews
- Fluorinated poly(N-vinylcarbazole) host for triplet energy confinement on phosphorescent emitter in organic light-emitting diodes, Y. Mizuno et al., MRS Online Proceedings Library, 1197, 19–24(2009); DOI: 10.1557/PROC-1197-D02-08.
- Antimicrobial potential of carbazole derivatives, V. Kadnor, Croat. Chem. Acta, 95(2), 39–48(2022); DOI: 10.5562/cca3899.
- Photophysical tuning of σ-SiH copper-carbazolide complexes to give deep-blue emission, A. Brannan et al., Inorg. Chem., 59(1), 315–324(2020); DOI: 10.1021/acs.inorgchem.9b02409.
