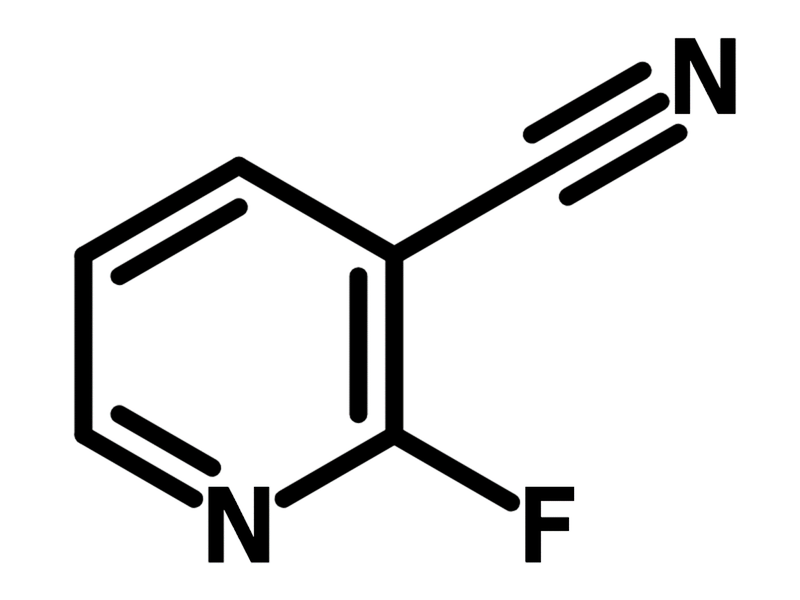
2-Fluoropyridine-3-carbonitrile
CAS Number 3939-13-7
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated nicotinonitrile building block
Used for the synthesis of bicyclic heterocycles in medicinal chemistry
Specifications | MSDS | Literature and Reviews
2-Fluoropyridine-3-carbonitrile (CAS number 3939-13-7), also named as 2-fluoronicotinonitrile, is a nicotinonitrile building block featuring a fluoride substituent at 2-position. 2-Fluoropyridine-3-carbonitrile commonly serves as a synthesis precursor for active pharmaceutical ingredients (APIs), such as niflumic acid that is used for joint and muscular pain relief. Pyrazopyridine, a bicyclic heterocycle can be synthesised from 2-fluoropyridine-3-carbonitrile. The reaction begins with a nucleophilic aromatic substitution of hydrazine, followed by a pyrazole cyclisation reaction.
Additionally, 2-fluoropyridine-3-carbonitrile can be modified through Minisci reaction for meta- and para-alkylation.
Multiple functional groups
For facile synthesis
Fluorinated hydroxybenzoic acid building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 3939-13-7 |
| Chemical Formula | C6H3FN2 |
| Full Name | 2-Fluoronicotinonitrile |
| Molecular Weight | 122.10 g/mol |
| Synonyms | 3-Cyano-2-fluoropyridine |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Nicotinonitrile building blocks, APIs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 28 °C – 33 °C |
| Appearance | White crystals |
MSDS Documentation
 2-Fluoropyridine-3-carbonitrile MSDS Sheet
2-Fluoropyridine-3-carbonitrile MSDS Sheet
Literature and Reviews
- Antiproliferative activity of some newly synthesized substituted pyridine candidates using 4‑(aryl)-6-(naphthalen-1-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile as synthon, A. El-Sayed et al., ACS Omega, 6, 7147–7156(2021); DOI: 10.1021/acsomega.1c00202.
- Second-generation CD73 inhibitors based on a 4,6-biaryl-2-thiophyridine scaffold, R. Ghoteimi et al., ChemMedChem, 18, e202200594(2023); DOI: 10.1002/cmdc.202200594.
- New reactions of 5-amino-3-(cyanomethyl)-1H-pyrazole-4-carbonitrile, V. Dotsenko et al., Chem. Proc., 3, 23(2023); DOI: 10.3390/ecsoc-24-08398.
